Abstract
The Nazarov cyclization is one of the most powerful tools for the stereoselective synthesis of various cyclopentenone scaffolds. Therefore, developing the new classes substrate of Nazarov reaction is an important endeavor in synthetic chemistry. Herein, we report enyne diketones, enables diastereo- and enantioselective construction of chiral allene cyclopentenones in moderate to good yields with good enantioselectivities (up to 97% ee). Importantly, it is a typical example for asymmetric synthesis of cyclopentanones with allene moiety using Nazarov cyclisation. Mechanistic studies indicate that this metal-organo relay catalysis protocol involves a rhodium-catalyzed tandem oxonium ylide formation/[2,3]-sigmatropic rearrangement/reverse benzylic acid rearrangement, followed by organo-catalyzed asymmetric Nazarov cyclization/alkyne-to-allene isomerization to give the final chiral allene cyclopentenones.
Similar content being viewed by others
Introduction
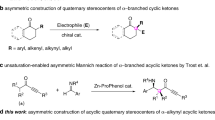
Enantiopure cyclopentenones are significant structural motifs that are widely present in numerous natural products and bio-significant molecules1,2,3,4. For example, some representative chiral cyclopentenones bearing 5-hydroxy-quaternary stereocenter scaffolds are illustrated in Fig. 1a5,6,7,8,9,10. Nazarov cyclization, one of the few 4π electrocyclic reactions that can be controlled in an asymmetric fashion by chiral catalysts, is considered to be one of the most direct and convenient methods to these important compounds in recent years1,2,3,4,11,12,13,14. Therefore, extensive efforts have been devoted to catalytic asymmetric versions of the Nazarov cyclizations. Scince Trauner’s pioneering the first asymmetric Nazarov cyclization in 200315, divinyl ketones became the most active research substrates for chemists over the past decades (Fig. 1b). The contribution works for asymmetric Nazarov cyclization of divinyl ketones includes the groups of Trauner15,16, Aggarwal17, Frontier18, Rueping19,20, Togni21, Tius22,23, Rawal24, Tang25,26, Zhou27, List28, Zhu29 and so on (Fig. 1b). In contrast, the other types substrate of asymmetric Nazarov cyclization are rarely reported. In 2010, Tius and co-workers designed a vinyl diketoesters substrate, which undergoes asymmetric Nazarov reactions to provide chiral cyclopentenones catalyzed by primary amine–thiourea catalyst (Fig. 1c)30. Later, the same group also reported Pd-catalyzed enantioselectve Nazarov cyclization of vinyl diketoesters substrate31. Furthermore, the group of Frontier developed an impressive dienyl diketones substrate that has been successfully used in the Nazarov reaction (Fig. 1d), however, most of their works were focused on the recemic electrocyclic reaction, few examples involving aysmmetric versions32,33,34,35.
Despite the above progress have been made, the substrate classes in asymmetric Nazarov cyclization remains quite limited. As a results, the diversity of the chiral cyclopentenones that can not be obtained. For example, the chiral cyclopentenone which bearing allene moiety can not be prepared by the reported Nazarov cyclization yet. In this regard, developing new classes substrate and catalytic strategies for asymmetric Nazarov cyclization to construction novel chiral cyclopentenones are highly desirable.
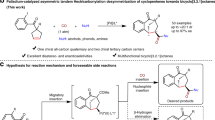
Herein, we report a metal-organo relay catalysis approach for the construction of chiral allene cyclopentenones in high diastereo- and enantioselectivities from 1,4-enyne alcohols with diazo compounds (Fig. 2a). The intermediate enyne diketones play a key role in the process of asymmetric Nazarov cyclization to form target compounds.
Based on literature reports30,31,32,33,34,35,36 and the control experiments and mechanism studies, the possible reaction mechanism of our design has been proposed (Fig. 2b). The formation of chiral allene cyclopentenone might proceed via a sequential process. First, the reaction between rhodium catalyst and diazoacetate 1 generates rhodium carbene species Int-1. Nucleophilic addition of 2 to Int-1 affords ylide intermediate Int-2, which undergoes a [2,3]-sigmatropic rearrangement yields Int-3. This intermediate would form 1,2-diketone-enyne 3 via reverse benzilic acid rearrangement37,38. Exposing 3 to a chiral thiourea catalyst may generate intermediate Int-4 via hydrogen-bonding and keto-enolic tautomerism. Intramolecular Nazarov cyclization delivers the cyclopentenone alkyne intermediate Int-5 containing two adjacent stereogenic centers. Under such reaction conditions, alkyne-to-allene isomerization occurs and undergoes central-to-axial chirality transfer39,40,41 to give the final product (–)-4.
Results
Reaction optimization
Inspired by the seminal reports by Davies36,42 and in continuation with our research interest in metal-carbene chemistry43,44,45,46,47. We conducted the reaction of diazoacetate 1a with 1,4-enyne-3-ol 2a bearing both an allylic and a propargylic moiety in the presence of Rh2(OAc)4, an enyne diketone 3 was generated in 92% NMR yield, which indicated that a reverse benzylic acid rearrangement of Int-3 occurred under such reaction conditions (Fig. 2b)37,38. Furthermore, subjecting enyne diketone 3 to silica gel, the cyclopentenone 4 was isolated in nearly 10% yield, this result revealed that the Nazarov cyclization was occurred. These unexpected experimental results and unconventional allene cyclopentenone structure prompted us to further explore the reaction.
We began to thoroughly investigate the reaction. With a view to establishing the optimal reaction conditions, phenyl diazoacetate 1a and 1,4-enyne alcohol 2a were used as model substrates (Table 1). A variety of diazoesters 1b–1f instead of 1a were tested under Method A, and no better results obtained (Table 1, entries 2-6). Intensively screening of the reaction conditions revealed that, using [Rh2(OAc)4] (2 mol%) as the catalyst and hexane as the solvent (rt for 12 h), with addition of MgO and CH2Cl2, allene cyclopentenone 4 was obtained in 79% yield in single isomer (Method A, one-pot process, see Table 1 and Supplementary Table 1 for details). Screening of the solvents revealed that hexane was still the best one (Table 1, entries 1, 7 and 8). Further evaluation of other rhodium catalysts, such as Rh2(TFA)4 and Rh2(esp)4 gave inferior results (entries 9-10). When SiO2 was used instead of MgO, only 43% yield of 4 was obtained (entry 11).
Substrates scope of racemic version
Having established the optimal reaction conditions for diastereoselective synthesis (Method A), we next started to investigate the scope of reactions (Fig. 3, all examples dr >19:1). The scope of enyne alcohols 2 was first evaluated. Generally, the R3 moiety of aryl-1,4-enyne alcohols containing either an electron-donating or an electron-withdrawing group at the para- or ortho-position of the phenyl ring were well tolerated, giving the corresponding products (4-9) in 63-82% yields with excellent diastereoselectivity (>19:1). However, the yield decreased when phenyl ring bearing 4-methoxycarbonyl group 10. Reactions of 4-Ph-phenyl, 3-cholor-phenyl, 2-cholorphenyl and 2-naphyl substituted enyne alcohols with 1a proceeded smoothly to give the desired products (11–14) in good yields, with high diastereoselectivity. The substrates with alkylated R3 group such as n-butyl, 3-Cl-proparyl, cyclopropyl and tert-butyl was also compatible, providing moderate yields of the corresponding products 15–18 (66–72%, >19:1 dr). Next, different R1 and R2 substituents were also explored, delivering 19–23 in 61–83% yields with >19:1 dr values. Obviously, the increased steric hindrance resulted in decreased yields.
aMethod A: 1 (0.3 mmol, 1.5 eq.), 2 (0.2 mmol, 1eq.) and Rh2(OAc)4 (2 mol%) in hexane (2 mL) stirred at rt. for 12 h under N2. Then, to the solution were added MgO (200 mg, 50 eq.), CH2Cl2 (5 mL) and stirred at 40 oC for another 3 h. bIsolated yield, dr > 19:1 (determined by 1H NMR analysis). cSovent of first step: (toluene:hexane = 1:1, 2 mL) instead of hexane. d1 (2.0 eq.) was used.
Next, we further assessed the generality of diazo compounds 1. With respect to the aryl diazoacetates, both electron-donating groups and electron-withdrawing groups at the para-position of phenyl rings proceeded smoothly to deliver the desired products 24–26 and 28–31 in moderate to good yields. The use of 4-BocNH substituted phenyldiazoacetate gave 27 in 38% yield. Aryldiazoacetates bearing an electron-donating (-Me and -OMe) or electron-withdrawing group (-Cl) at the mata-position of the phenyl ring reacted well, affording the corresponding products 32–34 in 68–74% yields. However, the use of ortho-methyl-substituted phenyldiazoacetate only afforded the desired products 35 in 34% yield. Furthermore, the di- and tri-substituted phenyl diazoacetates were also tested, providing the desired products 36–39 in moderate to good yields, with execellent dr values. Moreover, 2-naphthyl and heterocycles, such as 1,3-benzodioxole, Boc-indole ring and 3-thiophene were all tolerated, affording the desired products 40–43 in good yields. However, pyridine diazoacetate also work and furnishing allene cyclopentenone 44 in 36% yield.
Optimization of enantioselective synthesis
With the successful distereoselective synthesis of allene cyclopentenone 4, we then wish to realize the enantioselective synthesis of chiral allene cyclopentenone 4 by establishing a highly effective asymmetric catalytic system. Intensive attempts on transition-metal catalysis failed to reach high enantioselectivity. We then turned out our attention to use organo catalysts. However, preliminary evaluation of chiral phosphoric acids led to recover the starting materials (see Supplementary Table 2 for details). Next, cinchona-based chiral bases were examined (Fig. 4). Further studies revealed that the use of quinidine Q1 gave 4 in 76% yield but almost no enantioselectivity, whereas Q2 led to poor yield and low ee. The use of bifunctional chiral squaramides Q3-Q4 showed low catalytic reactivity, affording both low yields and low ee values. Subsequent utilization of amine-thiourea catalyst Q5–Q6 gave promising results,
Conditions: 1a (0.3 mmol, 1.5 eq.), 2a (0.2 mmol, 1eq.) and Rh2(OAc)4 (2 mol%) in hexane (2 mL) stirred at rt. for 12 h under N2; Then, removing the solvent and DCM (2.0 mL), Q9 (10 mol%) were added. The mixture was stirred for another 72 h. Isolated yield for two steps. Ee values were determined using chiral HPLC.
leading to moderate yield with >60% enantioselectivity. To further improve the enantioselectivity, we synthesized trifunctional binaphthyl diamine catalyst Q748 and used it in this reaction. The initial test delivered 4 in 63% yield and 82% ee. Suprisingly, when we prepared a large amount of Q7 with a view to screening other reaction conditions, only trace amount of 4 with 52% ee was obtained. After careful analysis, we found that we actually used Q9 not Q7 in the first try because Q7 can be easily converted to Q9 at higher temperature during the preparation process. Likewise, cinchonidine derived thiourea Q8 afforded 4 in 66% yield with 78% ee, whereas hydroquinidine derived Q10 gave 46% yield of 4 with 72% ee. In addition, the catalyst Q11–Q20 were also examined, unfortunately, there are no better results were obtained (see Supplementary Table 2 for details). Given Q9 exhibited the best catalytic activity in this reaction, other factors were further investigated, and as shown in Table 2.
Further screening the solvents indicated that cyclopentyl methyl ether (CPME) was the best one (Table 2, entries 1-7, and see Supplementary Table 3 for details). In screening additives, we found that 4 Å MS could improve the enantioselectivity (entry 7–8). The examination of organo-catalyst loading shown that 15 mol% gave a better yield (entries 9–11). As a result, the optimal reaction conditions for asymmetric catalysis had been established (Method B): 1a (0.3 mmol, 1.5 eq.), 2a (0.2 mmol, 1eq.), and Rh2(OAc)4 (2 mol%) in hexane (2 mL) stirred at room temperature for 12 h. Then, removing the solvent and CPME (2.0 mL), Q9 (15 mol%) and 4 Å MS (40 mg) were added. The mixture was stirred for another 72 h.
Substrate scope
Having established the optimal reaction conditions for enantioselective synthesis (Method B), we next started to investigate the scope of reactions (Fig. 5). The scope of enyne alcohols 2 was first evaluated. Generally, the R3 moiety of aryl-1,4-enyne alcohols containing either an electron-donating or an electron- withdrawing group at the para- or ortho-position of the phenyl ring were well tolerated, giving the corresponding chiral products (–)-(4) to (–)-10) in 48-66% yields and with 87-97% ee. Reactions of 4-Ph-phenyl, 3-cholor-phenyl, 2-cholorphenyl and 2-naphyl substituted enyne alcohols with 1a proceeded smoothly to give the desired chiral products (–)-(11) to (–)-14 in good yields, and high ee values. The substrates with alkylated R3 group such as n-butyl, 3-Cl-proparyl, cyclopropyl and tert-butyl were also compatible, providing moderate yields of the corresponding products (–)-(15)-(–)18 (50-64%, 86-89% ee, >19:1 dr). Next, different R1 and R2 substituents were also explored, delivering (–)-(19) to (–)-23 in 47-76% yields with 81-97% ee. Obviously, the increased steric hindrance resulted in decreased yields and ee values. Next, we further assessed the generality of diazo compounds 1 (Fig. 5). With respect to the aryl diazoacetates, both electron-donating groups and electron-withdrawing groups at the para-position of phenyl rings proceeded smoothly to deliver the desired products (-)-24-(-)-31) in moderate to good yields with 86-91% ee for the corresponding chiral allenes. Aryldiazoacetates bearing an electron-donating (-Me and -OMe) or electron-withdrawing group (-Cl) at the mata-position of the phenyl ring reacted well, affording the corresponding products (–)-(32)-(–)-34) in 63-66% yields with 90-92% ee. However, the use of ortho-methyl-substituted phenyldiazoacetate only afforded the desired products (–)-35 in 28% yield with 80% ee, which probably attributed to the large steric hindrance of methyl group. Furthermore, the di- and tri-substituted phenyl diazoacetates were also tested, providing the desired products (–)-36-(–)-39 in moderate to good yields, with good ee values. Moreover, 2-naphthyl and heterocycles, such as 1,3-benzodioxole, Boc-indole ring and 3-thiophene were all tolerated, affording the desired products (–)-40-(–)-43 in good yields and good enantioselectivities (84-86% ee). The absolute configurations in these chiral allenes were established by X-ray single-crystal diffraction study49. It should be noted that the product (-)-27 and (-)-44 were not obtained in the optimal asymmetric conditions (Method B).
aMethod B: 1 (0.3 mmol, 1.5 eq.), 2 (0.2 mmol, 1eq.), and Rh2(OAc)4 (2 mol%) in hexane (2 mL) stirred at room temperature for 12 h. Then, removing the solvent and CPME (2.0 mL), Q9 (15 mol%) and 4 Å MS (40 mg) were added. The mixture was stirred for another 72 h. bIsolated yields; all examples dr >19:1; the ee values were determined by chiral HPLC analysis. cMixed solvent (toluene/hexane = 1:3, 2 mL) instead of hexane in first step. dThe second step of method B for 96 h, and then the MgO (400 mg) was added and stirred for another 5 h.
Control experiments and mechanism studies
To gain insight into the reaction mechanism, control experiments were performed (Fig. 6). First, the reactions of 1a with 2 v and 2w under rhodium catalysis furnished the corresponding 1,2-diketones 45 and 46 in 71% and 58% yields, respectively (Fig. 6a, b). However, the use of 2x as substrate to reaction with 1a, as we expected, no corresponding diketone 47 was observed, instead, the O-H insertion product 47’ was obtained in 68% yield (Fig. 6c). These results probably suggested that the π-σ(C-OH)-π is essential structure of alcohol to occur the sequential ylide formation/[2,3]-sigmatropic rearrangement and reverse benzylic acid rearrangement to form the 1,2-diketone. Next, we conducted the deuterium experiment for further insight into mechanism process. The addition of D2O to the reaction of 1a and 2a in the conditions of method B and method A, gave D-4 with 70% and 30% deuterium labeling, respectively (Fig. 6d). The reason of Q9 give the better deuterium labeling ratio than MgO probably due to the Q9 has higher ability to stable intermediate anion, and the result also supported that the proton transfer has occurred in the path from alkyne to allene. It is worth noting that both alkyne-containing alcohol (–)-36ʹ and allenol (–)-36 were formed during the enantioselective cyclo-isomerization of diketone 48 (Scheme 6e). The yields of (–)-36ʹ and (–)-36 were time-depending. The amount of (–)-36 increased by prolonging the reaction to 92 h. In contrast, the yield of (–)-36ʹ increased to 35% within 48 h and then decreased with longer reaction time (Fig. 6e). To further validate the prerequisite formation of (–)-36ʹ, subjecting (–)-36ʹ to SiO2 or organic catalyst Q9, both of them are led to high yield of (–)-36 with enantioselectivity retention (Fig. 6f). Indeed, in the presence of base, such as Et3N, the isomerization from alkyne-to-allene also proceeded smoothly. Even absence of catalyst, the isomerization of (–)-36ʹ also could occur when stirred in the solvent of dichloromethane (Fig. 6g).
a 1,4-enyne allylic alcohol as substrate. b 1,4-dienyl allylic alcohol as substrate. c monoallylic alcohol as substrate. d Deuterium experiment. e The coversation of compound 48 in different time periods. f Both Q9 and SiO2 accelerate (–)–36‘ conversion to allene (–)–36. g (–)–36‘ in the condition of base or CH2Cl2.
Gram scale and synthetic application
Further derivatization reactions were performed to demonstrate the promising synthetic practicality of this method (Fig. 7). Treatment of 1a with 2b, followed by addition of 1,2-diaminobenzene gave heterocycle 49 in 89% yield (see Supplementary for details). The structure of 49 was determined by single-crystal X-ray diffraction, which also confirmed the structures of enyne diketone intermediates. To further assess the utility of this tandem annulation, a gram scale synthesis was conducted, affording (–)-20 in 68% yield with no significant ee erosion (1.112 g) (Fig. 7a). Then, conversion reactions of (–)-20 were conducted. Subjecting (–)-20 with LiAlH4 yielded 1,2-diol 50 in 92% yield with 94% ee. Addition of methyl Grignard reagent to (–)-20 led to tertiary alcohol 51 in a good yield with 95% ee. Finally, selective hydrogenation of allene moiety provided 52 in 96% yield and 96% ee. Interestingly, the reaction of 20 with TfOH in dichloromethane, a racemic condensed polycyclic compound 53 was generated (Fig. 7b).
Discussion
In this work, we describle an efficient protocol to construct chiral allene cyclopentenones from 1,4-enyne alcohols with diazo compounds via metal-organo relay catalysis strategy. The intermediate enyne diketones represent an underdevelop classes of substate in asymmetric Nazarov cyclization.
Methods
General procedure for the synthesis and experiment data of (±)-4-44 and (-)-4-(-)43
Method A (one-pot process for racemic products): To the tube was added freshly distilled hexane (2.0 mL), diazo 1 (0.3 mmol), enyn-3-ol 2 (0.2 mmol) and then Rh2(OAc)4 (2.6 mg, 2 mol%) was added to the mixture solution. The tube was sealed and stirred at rt for 12 h. And then MgO (400 mg, 50 eq.) and CH2Cl2 (5 mL) was added to the mixture solution, and stirred at 40 oC for another 3 h. Then the mixture solution was filtrated by diatomite, and wash with EtOAc (10*3). The filtrate was concentrated by rotary evaporation. The crude product was purified by silica gel column chromatography corresponding eluent to afford the desired products (±)-4-44.
Method B (for asymmetric version): To the dried tube was added freshly distilled hexane (2.0 mL), diazo 1 (0.3 mmol), enyn-3-ol 2 (0.2 mmol), and then Rh2(OAc)4 (2.6 mg, 2 mol%) was added to the mixture solution. The tube was sealed and stirred at rt. for 12 h. The solvent was removed directly; and then, the CPME (2.0 mL), Q9 (15 mol%) and 4ÅMs (40 mg) was added to the tube, stirred at rt. for another 72 h. The solution was concentrated under reduced pressure and purified by flash chromatography corresponding eluent to afford the desired products (-)-4-43.
Data availability
All data generated in this study are provided in the Airticle and Supplementary Information as well as from the corresponding author on request. The X-ray crystallographic data used in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code CCDC 2059825 for (–)-20 and CCDC 2059828 for 49, CCDC 2133232 for 53. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Roche, S. P. & Aitken, D. J. Chemistry of 4-hydroxy-2-cyclopentenone derivatives. Eur. J. Org. Chem. 2010, 5339–5358 (2010).
Simeonov, S. P., Nunes, J. P. M., Guerra, K., Kurteva, V. B. & Afonso, C. A. M. Synthesis of chiral cyclopentenones. Chem. Rev. 116, 5744–5893 (2016).
Vinogradov, M. G., Turova, O. V. & Zlotin, S. G. Nazarov reaction: current trends and recent advances in the synthesis of natural compounds and their analogs. Org. Biomol. Chem. 15, 8245–8269 (2017).
Frontier, A. J. & Hernandez, J. J. New twists in Nazarov cyclization chemistry. Acc. Chem. Res 53, 1822–1832 (2020).
Pohmakotr, M., Kambutong, S., Tuchinda, P. & Kuhakarn, C. Asymmetric synthesis of pentenomycin I, epipentenomycin I, and their analogs. Tetrahedron 64, 6315–6323 (2008).
Xu, G. et al. Przewalskin B, a Novel diterpenoid with an unprecedented skeleton from salvia przewalskii Maxim. Org. Lett. 9, 291–293 (2006).
Lv, J.-H. et al. Novel 2,5-diarylcyclopentenone derivatives from the wild edible mushroom paxillus involutus and their antioxidant activities. J. Agric. Food Chem. 69, 5040–5048 (2021).
Fukuda, T., Nagai, K. & Tomoda, H. (±)-Tylopilusins, diphenolic metabolites from the fruiting bodies of tylopilus eximius. J. Nat. Prod. 75, 2228–2231 (2012).
Otaka, J. et al. Hitoyol A and B, two norsesquiterpenoids from the basidiomycete coprinopsis cinerea. Org. Lett. 19, 4030–4033 (2017).
Chianese, G. et al. Spirocurcasone, a diterpenoid with a novel carbon skeleton from Jatropha curcas. Org. Lett. 13, 316–319 (2011).
Frontier, A. J. & Collison, C. The Nazarov cyclization in organic synthesis. recent advances. Tetrahedron 61, 7577–7606 (2005).
Shimada, N., Stewart, C. & Tius, M. A. Asymmetric Nazarov cyclizations. Tetrahedron 67, 5851–5870 (2011).
Vaidya, T., Eisenberg, R. & Frontier, A. J. Catalytic Nazarov cyclization: the state of the art. ChemCatChem 3, 1531–1548 (2011).
West, F. G., Scadeng, O., Wu, Y. K., Fradette, R. J. & Joy, S. The Nazarov cyclization. In comprehensive organic synthesis II, 2nd ed (ed. Knochel, P.) 827–866 (Elsevier: Amsterdam, 2014).
Liang, G., Gradl, S. N. & Trauner, D. Efficient Nazarov cyclizations of 2-alkoxy-1,4-pentadien-3-ones. Org. Lett. 5, 4931–4934 (2003).
Liang, G. & Trauner, D. Enantioselective Nazarov reactions through catalytic asymmetric proton transfer. J. Am. Chem. Soc. 126, 9544–9545 (2004).
Aggarwal, V. K. & Belfield, A. J. Catalytic asymmetric Nazarov reactions promoted by chiral Lewis acid complexes. Org. Lett. 5, 5075–5078 (2003).
He, W., Sun, X. & Frontier, A. J. Polarizing the Nazarov cyclization: efficient catalysis under mild conditions. J. Am. Chem. Soc. 125, 14278–14279 (2003).
Rueping, M., Ieawsuwan, W., Antonchick, A. P. & Nachtsheim, B. J. Chiral Brønsted acids in the catalytic asymmetric Nazarov cyclization-the first enantioselective organocatalytic electrocyclic reaction. Angew. Chem. Int. Ed. 46, 2097–2100 (2007).
Raja, S., Ieawsuwan, W., Korotkov, V. & Rueping, M. Asymmetric Brønsted acid-catalyzed Nazarov cyclization of acyclic α-alkoxy dienones. Chem. Asian J. 7, 2361–2366 (2012).
Walz, I. & Togni, A. Ni(II)-Catalyzed enantioselective Nazarov cyclizations. Chem. Commun. 36, 4315−4317 (2008).
Bow, W. F., Basak, A. K., Jolit, A., Vicic, D. A. & Tius, M. A. Enamine-iminium ion Nazarov cyclization of α-ketoenones. Org. Lett. 12, 440–443 (2010).
Jolit, A., Walleser, P. M., Yap, G. P. A. & Tius, M. A. Catalytic enantioselective Nazarov cyclization: construction of vicinal all-carbon-atom quaternary stereocenters. Angew. Chem., Int. Ed. 53, 6180–6183 (2014).
Hutson, G. E., Turkmen, Y. E. & Rawal, V. H. Salen promoted enantioselective Nazarov cyclizations of activated and unactivated dienones. J. Am. Chem. Soc. 135, 4988–4991 (2013).
Cao, P. et al. Asymmetric Nazarov reaction catalyzed by chiral tris(oxazoline)/copper(II). Angew. Chem., Int. Ed. 49, 4463–4466 (2010).
Xu, Z., Ren, H., Wang, L. & Tang, Y. Efficient catalytic enantioselective Nazarov cyclizations of divinyl ketoesters. Org. Chem. Front. 2, 811–814 (2015).
Wang, G.-P., Chen, M.-Q., Zhu, S.-F. & Zhou, Q.-L. Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids. Chem. Sci. 8, 7197–7202 (2017).
Ouyang, J., Kennemur, J. L., De, C. K., Fares, C. & List, B. Strong and confined acids enable a catalytic asymmetric Nazarov cyclization of simple divinyl ketones. J. Am. Chem. Soc. 141, 3414–3418 (2019).
Cao, J. et al. Enantioselective silicon-directed Nazarov cyclization. J. Am. Chem. Soc. 143, 6962–6968 (2021).
Basak, A. K., Shimada, N., Bow, W. F., Vicic, D. A. & Tius, M. A. An organocatalytic asymmetric Nazarov cyclization. J. Am. Chem. Soc. 132, 8266–8267 (2010).
Kitamura, K. et al. Enantioselective palladium(0)-catalyzed Nazarov-type cyclization. Angew. Chem. Int. Ed. 54, 6288–6291 (2015).
Brooks, J. L., Caruana, P. A. & Frontier, A. J. Conjugate addition-initiated Nazarov cyclization. J. Am. Chem. Soc. 133, 12454–12457 (2011).
Brooks, J. L. & Frontier, A. J. Cyclization cascades initiated by 1,6-conjugate addition. J. Am. Chem. Soc. 134, 16551–16553 (2012).
Jacob, S. D., Brooks, J. L. & Frontier, A. J. No acid required: 4π and 6π electrocyclization reactions of dienyl diketones for the synthesis of cyclopentenones and 2H-pyrans. J. Org. Chem. 79, 10296–10302 (2014).
Huang, Y.-W. & Frontier, A. J. Enantioselective Nazarov cyclization catalyzed by a cinchona alkaloid derivative. Tetrahedron Lett. 56, 3523–3526 (2015).
Li, Z. & Davies, H. M. L. Enantioselective C−C bond formation by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and allylic alcohols. J. Am. Chem. Soc. 132, 396–401 (2010).
Becker, H.-D., Sörensen, H. & Hammarberg, E. 9,9’-Anthril (di-9-anthrylethanedione). Tetrahedron Lett. 30, 989–992 (1989).
Burke, A. J. & Marques, C. S. Mechanistic and synthetic aspects of the benzilic acid and ester rearrangements. Mini-Rev. Org. Chem. 4, 310–316 (2007).
Dabrowski, J. A., Haeffner, F. & Hoveyda, A. H. Combining NHC–Cu and brønsted base catalysis: enantioselective allylic substitution/conjugate additions with alkynylaluminum reagents and stereospecific isomerization of the products to trisubstituted allenes. Angew. Chem. Int. Ed. 52, 7694–7699 (2013).
Wei, X.-F. et al. Catalytic regio- and enantioselective proton migration from skipped enynes to allenes. Chem 5, 585–599 (2019).
Luis, J. et al. Highly stereoselective synthesis of tertiary propargylic centers and their isomerization to enantiomerically enriched allenes. Chem. Eur. J. 18, 9775–9779 (2012).
Li, Z. et al. Scope and mechanistic analysis of the enantioselective synthesis of allenes by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and propargylic alcohols. J. Am. Chem. Soc. 134, 15497–15504 (2012).
Zhu, C., Xu, G. & Sun, J. Gold-catalyzed formal [4+1]/[4+3] cycloadditions of diazo esters with triazines. Angew. Chem. Int. Ed. 55, 11867–11871 (2016).
Liu, K., Xu, G. & Sun, J. Gold-catalyzed stereoselective dearomatization/metal-free aerobic oxidation: access to 3-substituted indolines/oxindoles. Chem. Sci. 9, 634–639 (2018).
Wang, K. et al. Rhodium-catalyzed regioselective N2-alkylation of benzotriazoles with diazo compounds/enynones via a nonclassical pathway. Angew. Chem. Int. Ed. 57, 12489–12493 (2018).
Xu, G. et al. Access to N-substituted 2-pyridones by catalytic intermolecular dearomatization and 1,4-acyl transfer. Angew. Chem. Int. Ed. 58, 1980–1984 (2019).
Wang, K. et al. Chemo- and enantioselective insertion of furyl carbene into the N−H bond of 2-pyridones. Angew. Chem. Int. Ed. 60, 16942–16946 (2021).
Tan, B., Candeias, N. R. & Barbas, C. F. III Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst. Nat. Chem. 3, 473–477 (2011).
CCDC 2059825 for (–)-20 and CCDC 2059828 for 49, CCDC 2133232 for 53.
Acknowledgements
We thank the National Natural Science Foundation of China (21901022, 21971026, 22171028) and the Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (BM2012110) for their financial support.
Author information
Authors and Affiliations
Contributions
S.T. conceived the idea, performed the most experiments and wrote the draft manuscript; P.Z. assisted in some experiments and helped in synthesis of substrates. Y.S. collected and analyzed the data; J.S. guided this project and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, S., Zhang, P., Shao, Y. et al. Enyne diketones as substrate in asymmetric Nazarov cyclization for construction of chiral allene cyclopentenones. Nat Commun 13, 3146 (2022). https://doi.org/10.1038/s41467-022-30846-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30846-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.