Abstract
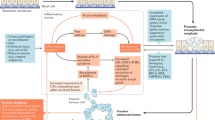
Peyronie’s disease, a fibroinflammatory disorder, detrimentally impacts the sexual well-being of men and their partners. The manifestation of fibrotic plaques within penile tissue, attributed to dysregulated fibrogenesis, is pathognomonic for this condition. The onset of fibrosis hinges on the perturbation of the equilibrium between matrix metalloproteinases (MMPs), crucial enzymes governing the extracellular matrix, and tissue inhibitors of MMPs (TIMPs). In the context of Peyronie’s disease, there is an elevation in TIMP levels coupled with a decline in MMP levels, culminating in fibrogenesis. Despite the scant molecular insights into fibrotic pathologies, particularly in the context of Peyronie’s disease, a comprehensive literature search spanning 1995 to 2023, utilizing PubMed Library, was conducted to elucidate these mechanisms. The findings underscore the involvement of growth factors such as FGF and PDGF, and cytokines like IL-1 and IL-6, alongside PAI-1, PTX-3, HIF, and IgG4 in the fibrotic cascade. Given the tissue-specific modulation of fibrosis, comprehending the molecular underpinnings of penile fibrosis becomes imperative for the innovation of novel and efficacious therapies targeting Peyronie’s disease. This review stands as a valuable resource for researchers and clinicians engaged in investigating the molecular basis of fibrotic diseases, offering guidance for advancements in understanding Peyronie’s disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chung E, Ralph D, Kagioglu A, et al. Evidence-based management guidelines on Peyronie’s disease. J Sex Med. 2016;13:905–23.
Patel DP, Christensen MB, Hotaling JM, Pastuszak AW. A review of inflammation and fibrosis: implications for the pathogenesis of Peyronie’s disease. World J Urol. 2020;38:253–61.
Bilgutay AN, Pastuszak AW. Peyronie’s disease: a review of etiology, diagnosis, and management. Curr Sex Health Rep. 2015;7:117–31.
Kuehhas FE, Weibl P, Georgi T, Djakovic N, Herwig R. Peyronie’s disease: nonsurgical therapy options. Rev Urol. 2011;13:139–46.
Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–84.
Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol. 2014;10:700–11.
Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, et al. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int. 2001;59:579–92. https://doi.org/10.1046/j.1523-1755.2001.059002579.x.
Weng CM, Li Q, Chen KJ, Xu CX, Deng MS, Li T, et al. Bleomycin induces epithelial-to-mesenchymal transition via bFGF/PI3K/ESRP1 signaling in pulmonary fibrosis. Biosci Rep. 2020;40:BSR20190756 https://doi.org/10.1042/BSR20190756.
Maccarinelli F, Bugatti M, Churruca Schuind A, Ganzerla S, Vermi W, Presta M, et al. Endogenous long Pentraxin 3 exerts a protective role in a murine model of pulmonary fibrosis. Front Immunol. 2021;12:617671 https://doi.org/10.3389/fimmu.2021.617671.
Roeb E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018;68:463–73.
Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123.
Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12:233 https://doi.org/10.1186/gb-2011-12-11-233.
Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33.
Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811.
Schmierer B, Hill CS. TGFβ–SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82.
Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404.
El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF. Peyronie’s disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997;158:1391–4.
Haag SM, Hauck EW, Szardening-Kirchner C, Diemer T, Cha ES, Weidner W, et al. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie’s disease. Eur Urol. 2007;51:255–61. https://doi.org/10.1016/j.eururo.2006.05.002.
Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol. 2002;13:1464–72. https://doi.org/10.1097/01.asn.0000014252.37680.e4.
Magee TR, Qian A, Rajfer J, Sander FC, Levine LA, Gonzalez-Cadavid NF. Gene expression profiles in the Peyronie’s disease plaque. Urology. 2002;59:451–7.
Hauck EW, Hauptmann A, Schmelz HU, Bein G, Weidner W, Hackstein H. Prospective analysis of single nucleotide polymorphisms of the transforming growth factor beta-1 gene in Peyronie’s disease. J Urol. 2003;169:369–72. https://doi.org/10.1016/S0022-5347(05)64129-8.
Jung KH, Ryu YL, Lee HS, Lee H, Son MK, Yan HH, et al. A novel PI3K inhibitor alleviates fibrotic responses in fibroblasts derived from Peyronie’s plaques. Int J Oncol. 2013;42:2001–8.
Song KM, Chung DY, Choi MJ, Ghatak K, Minh NN, Limanjaya A, et al. Vactosertib, a novel, orally bioavailable activin receptor-like kinase 5 inhibitor, promotes regression of fibrotic plaques in a rat model of Peyronie’s disease. World J Men’s Health. 2020;38:552–63. https://doi.org/10.5534/wjmh.190071.
Carthy JM, Sundqvist A, Heldin A, van Dam H, Kletsas D, Heldin CH, et al. Tamoxifen inhibits TGF-β-Mediated activation of myofibroblasts by blocking non-smad signaling through ERK1/2. J Cell. Physiol. 2015;230:3084–92. https://doi.org/10.1002/jcp.25049.
Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie’s disease. J Urol. 1999;162:2003–5. https://doi.org/10.1016/S0022-5347(05)68087-1.
Shindel AW, Lin G, Ning H, Banie L, Huang YC, Liu G, et al. Pentoxifylline attenuates transforming growth factor-β1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: impact on extracellular matrix. J Sex Med. 2010;7:2077–85. https://doi.org/10.1111/j.1743-6109.2010.01790.x.
Smith JF, Shindel AW, Huang YC, Clavijo RI, Flechner L, Breyer BN, et al. Pentoxifylline treatment and penile calcifications in men with Peyronie’s disease. Asian J Androl. 2011;13:322–5. https://doi.org/10.1038/aja.2010.117.
Rose-John S, Winthrop K, Calabrese L.The role of IL-6 in host defence against infections: immunobiology and clinical implications.Nat Rev Rheumatol. 2017;13:399–409.
Calabrese LH, Rose-John S.IL-6 biology: implications for clinical targeting in rheumatic disease.Nat Rev Rheumatol. 2014;10:720–7.
Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, et al. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol. 2003;29:490–8.
Denton CP, Ong VH, Xu S, Chen-Harris H, Modrusan Z, Lafyatis R, et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Annal Rheum Dis. 2018;77:1362–71.
Atar Arda, et al. Role of interleukin-6 and pentraxin 3 as an early marker in Peyronie’s disease. Kaohsiung J Med Sci. 2017;33:195–200.
Watanabe MS, Theodoro TR, Coelho NL, Mendes A, Leonel MLP, Mader AM, et al. Extracellular matrix alterations in the Peyronie’s disease. J Adv Res. 2017;8:455–61.
Zimmermann RP, Feil G, Bock C, Hoeltl L, Stenzl A. Significant alterations of serum cytokine levels in patients with Peyronie’s disease. Int. braz J Urol. 2008;34:457–66.
Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72–91. https://doi.org/10.1111/j.1755-5922.2010.00171.x.
Ha H, Oh EY, Lee HB. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol. 2009;5:203–11. https://doi.org/10.1038/nrneph.2009.15.
Davila HH, et al. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie’s disease.”. BJU Int. 2003;91:830–8.
Davila HugoH, et al. “Peyronie’s disease associated with increase in plasminogen activator inhibitor in fibrotic plaque.”. Urology. 2005;65:645–8.
Garlanda C, Bottazzi B, Magrini E, Inforzato A, Mantovani A. PTX3, a humoral pattern recognition molecule, in innate immunity, tissue repair, and cancer. Physiol Rev. 2018;98:623–39. https://doi.org/10.1152/physrev.00016.2017.
Gorka-Dynysiewicz J, Pazgan-Simon M, Zuwala-Jagiello J. Pentraxin 3 detects clinically significant fibrosis in patients with chronic viral hepatitis C. BioMed Res Int. 2019;2019:2639248 https://doi.org/10.1155/2019/2639248.
Mamer SB, Chen S, Weddell JC, Palasz A, Wittenkeller A, Kumar M, et al. Discovery of high-affinity PDGF-VEGFR interactions: redefining RTK dynamics. Sci Rep. 2017;7:1–14.
Zou X, Tang XY, Qu ZY, Sun ZW, Ji CF, Li YJ, et al. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: a review. Int J Biol Macromol. 2022;202:539–57.
Zhao Tieqiang, et al. “Platelet-derived growth factor-D promotes fibrogenesis of cardiac fibroblasts.”. Am J Physiol-Heart Circ Physiol. 2013;304:H1719–26.
Lucattelli M, Lunghi B, Fineschi S, Mirone V, di Villa Bianca RDE, Longo N, et al. A new mouse model of Peyronie’s disease: an increased expression of hypoxia-inducible factor-1 target genes during the development of penile changes. Int J Biochem Cell Biol. 2008;40:2638–48.
Gentile V, et al. “Ultrastructural and immunohistochemlcal Characterization of the Tunica Albuginea in Peyronie’s Disease and Veno‐occlusive Dysfunction.”. J Androl. 1996;17:96–103.
Aversa A, et al. “Platelet-derived growth factor (PDGF) and PDGF receptors in rat corpus cavernosum: changes in expression after transient in vivo hypoxia.”. J Endocrinol. 2001;170:395–402.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39.
Gokce A, Abd Elmageed ZY, Lasker GF, Bouljihad M, Kim H, Trost LW, et al. Adipose tissue–derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie’s disease. Andrology. 2014;2:244–51.
Campbell J, DeYoung L, Chung E, Brock G. 022 matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in the pathogenesis of Peyronie’s Disease. J Sex Med. 2016;13:S11.
Castiglione F, Hedlund P, Weyne E, Hakim L, Montorsi F, Bivalacqua TJ, et al. Intratunical injection of human adipose tissue–derived stem cells restores collagen III/I ratio in a rat model of chronic Peyronie’s disease. Sex Med. 2019;7:94–103.
Del Carlo M, Cole AA, Levine LA. Differential calcium independent regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by interleukin-1β and transforming growth factor-β in Peyronie’s plaque fibroblasts. J Urol. 2008;179:2447–55.
Karavitakis Markos, et al. “The relationship between androgens, regulators of collagen metabolism, and Peyronie’s disease: a case control study.”. J Sex Med. 2010;7:4011–7.
Yang Q, Chen W, Han D, Zhang C, Xie Y, Sun X, et al. Intratunical injection of human urine‐derived stem cells derived exosomes prevents fibrosis and improves erectile function in a rat model of Peyronie’s disease. Andrologia. 2020;52:e13831.
Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–83.
Roth KJ, Copple BL. Role of hypoxia-inducible factors in the development of liver fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1:589–97.
Cui Y, Wang Y, Men C, Wu J, Liu L. Bioinformatics-Based identification of potential hypoxia-related genes associated with Peyronie’s Disease. Am J Men’s Health. 2022;16:15579883221111720.
Hu J, Wang W, Zhang F, Li PL, Boini KM, Yi F, et al. Hypoxia inducible factor-1α mediates the profibrotic effect of albumin in renal tubular cells. Sci Rep. 2017;7:15878.
Solakhan M, Kısacık B. Is Peyronie’s an IgG4-related disease? Eur J Rheumatol. 2021;8:27.
Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 2020;16:702–14.
Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62.
Shindel AW, Sweet G, Thieu W, Durbin-Johnson B, Rothschild J, Szabo R. Prevalence of Peyronie’s disease-Like symptoms in men presenting with Dupuytren contractures. Sex Med. 2017;5:e135–41.
Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280:36099–109.
Taguchi S, Azushima K, Yamaji T, Urate S, Suzuki T, Abe E, et al. Effects of tumor necrosis factor-α inhibition on kidney fibrosis and inflammation in a mouse model of aristolochic acid nephropathy. Sci Rep. 2021;11:23587.
Milenkovic U, Janky R, Hatzichristodoulou G, Van Renterghem K, Cellek S, Bivalacqua TJ, et al. Transcriptome-wide analysis of Peyronie’s disease plaques using RNA sequencing uncovers targetable signalling pathways for medical therapy. Eur Urol Suppl. 2019;18:e1245.
Pavone C, Caruana G, Abbadessa D, Scaduto G, Gambino G, Serretta V, et al. Cytokine gene expression in the tunica albuginea of patients with Peyronie’s disease. Pilot study with a control group. Urol J. 2012;79:189–96.
Milenkovic U, Ilg MM, van Renterghem K, De Ridder D, Albersen M. 277 Interplay between TNFa and TGF-B1 in a cell culture model of Peyronie’s Disease. J Sex Med. 2018;15:S234–S234.
Nanchahal J, Ball C, Rombach I, Williams L, Kenealy N, Dakin H, et al. Anti-tumour necrosis factor therapy for early-stage Dupuytren’s disease (RIDD): a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2022;4:e407–16.
Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18.
Gelfand RA, Vernet D, Kovanecz I, Rajfer J, Gonzalez‐Cadavid NF. The transcriptional signatures of cells from the human Peyronie’s disease plaque and the ability of these cells to generate a plaque in a rat model suggest potential therapeutic targets. J Sex Med. 2015;12:313–27.
Hernandez DM, Kang JH, Choudhury M, et al. IPF pathogenesis is dependent upon TGFβ induction of IGF-1. FASEB J. 2020;34:5363–88. https://doi.org/10.1096/fj.201901719RRqiyu.
Borthwick, LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. In Seminars in immunopathology. Springer Berlin Heidelberg; 2016. p. 517–34.
Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J.Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis.J Clin Investig. 2001;107:1529–36.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37.
Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie’s disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010;7:215–21.
Toblli JE, Ferrini MG, Cao G, Vernet D, Angerosa M, Gonzalez-Cadavid NF. Antifibrotic effects of pioglitazone on the kidney in a rat model of type 2 diabetes mellitus. Nephrol Dialysis Transplant. 2009;24:2384–91.
Khor YH, Adegunsoye A. Inhaled nitric oxide for fibrotic interstitial lung disease: a step forward. Annal Am Thoracic Soc. 2022;19:536–8. https://doi.org/10.1513/AnnalsATS.202110-1160ED.
Bivalacqua TJ, Champion HC, Leungwattanakij S, Yang DY, Hyun JS, Abdel‐Mageed AB. et al. Evaluation of nitric oxide synthase and arginase in the induction of a Peyronie’s‐like condition in the rat. J Androl. 2001;22:497–506.
Ferrini MG, Rivera S, Moon J, Vernet D, Rajfer J, Gonzalez-Cadavid NF. The genetic inactivation of inducible nitric oxide synthase (iNOS) intensifies fibrosis and oxidative stress in the penile corpora cavernosa in type 1 diabetes. J Sex Med. 2010;7:3033–44. https://doi.org/10.1111/j.1743-6109.2010.01884.x.
Vernet D, Ferrini MG, Valente EG, Magee TR, Bou-Gharios G, Rajfer J, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric Oxide. 2002;7:262–76. https://doi.org/10.1016/s1089-8603(02)00124-6.
Davila HH, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Gene transfer of inducible nitric oxide synthase complementary DNA regresses the fibrotic plaque in an animal model of Peyronie’s disease. Biol Reprod. 2004;71:1568–77.
Bivalacqua TJ, Champion HC, Mehta YS, et al. Adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to the penis improves age-related erectile dysfunction in the rat. Int J Impot Res. 2000;12:S8–17. https://doi.org/10.1038/sj.ijir.3900556.
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, et al. FGF/FGFR signaling in health and disease. Signal Transduct Targeted Ther. 2020;5:181.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev: Dev Biol. 2015;4:215–66.
Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–80.
Dolivo DM, Larson SA, Dominko T. Fibroblast growth factor 2 as an antifibrotic: antagonism of myofibroblast differentiation and suppression of pro-fibrotic gene expression. Cytokine Growth Factor Rev. 2017;38:49–58.
Mulhall JP, Thom J, Lubrano T, Shankey TV.Basic fibroblast growth factor expression in Peyronie’s disease.J Urol. 2001;165:419–23.
Mulhall JP, Branch J, Lubrano T, Shankey TV.Radiation increases fibrogenic cytokine expression by Peyronie’s disease fibroblasts.J Urol. 2003;170:281–4.
Chung E, De Young L, Brock GB. Rat as an animal model for Peyronie’s disease research: a review of current methods and the peer-reviewed literature. Int J Impot Res. 2011;23:235–41.
Stancik I, Schäfer R, Andrukhova O, Oeser R, Plas E, Pflüger H. Effect of transdermal electromotive drug therapy on fibrogenic cytokine expression in Peyronie’s disease. Urology. 2009;74:566–70.
Burgy O, Königshoff M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018;68-69:67–80. https://doi.org/10.1016/j.matbio.2018.03.017.
Dolmans GH, Werker PM, de Jong IJ, Nijman RJ, LifeLines Cohort Study, Wijmenga C, Ophoff RA. WNT2 locus is involved in genetic susceptibility of Peyronie’s disease. J Sex Med. 2012;9:1430–4.
Ten Dam EJP, van Driel MF, de Jong IJ, Werker PM, Bank RA. Glimpses into the molecular pathogenesis of Peyronie’s disease. Aging Male. 2020;23:962–70.
De Young LX, Bella AJ, O’Gorman DB, Gan BS, Lim KB, Brock GB.Protein biomarker analysis of primary Peyronie’s disease cells.J Sex Med. 2010;7:99–106.
Liu T, Shindel AW, Lin G, Lue TF. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Impot Res. 2019;31:170–6.
Gundogdu G, Nguyen T, Namasivayam A, Starek S, Gelman J, Mauney, JR. Characterization of a novel rabbit model of Peyronie’s disease. Int J Impot Res. 2023. https://doi.org/10.1038/s41443-023-00671-y.
Minor TX, Lin G, Jad A, Lin CS, Hayashi N, F. Lue T. 1058: the effect of pentoxifylline on cultured human tunical fibroblasts from patients with Peyronie’s Disease. J Urol. 2005;173:287–287.
Campbell J, DeYoung L, Chung E, Brock G. Mp89-20 traction applied to peyronie’s disease cells reduces cellular fibrosis. J Urol. 2016;195:e1144.
Geng Q, Wang F, Han Q, Chen SF, Ouyang B, Li Z, et al. Antioxidant mechanism of Xiaojin Pill (小金丸) for treatment of Peyronie’s disease in rats based on matrix metalloproteinases. Chin J Integr Med. 2019;25:671–6.
Author information
Authors and Affiliations
Contributions
Murat Gül had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Gül, Şahin. Acquisition of data: Babayev, Şahin, Cirigliano, Altıntaş. Analysis and interpretation of data: Preto, Şahin, Babayev. Drafting of the manuscript: Şahin, Babayev, Gül. Critical revision of the manuscript for important intellectual content: Falcone, Gül. Statistical analysis: Şahin, Altıntaş, Babayev. Obtaining funding: None. Administrative, technical, or material support: None. Supervision: Gül, Falcone. Other: None.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Şahin, A., Babayev, H., Cirigliano, L. et al. Unveiling the molecular Hallmarks of Peyronie’s disease: a comprehensive narrative review. Int J Impot Res (2024). https://doi.org/10.1038/s41443-024-00845-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-024-00845-2