Abstract
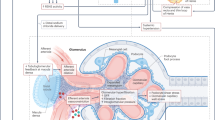
c-Jun N-terminal kinases (JNKs) are involved in the myocardial and aortic remodeling, increased arterial tone, and arterial blood pressure elevation associated with hypertension. The aim of the present study was to investigate the antihypertensive effect of a new JNK inhibitor, 1H-indeno[1,2-b]quinoxalin-11-one oxime sodium salt (IQ-1S), on spontaneously hypertensive rats (SHRs). Experiments were performed using normotensive Wistar-Kyoto (WKY) rats and SHRs. Experimental groups of SHRs received IQ-1S intragastrically for 6 weeks in daily doses of 5 and 50 mg/kg; experimental groups of WKY rats received 50 mg/kg IQ-1S according to the same regimen. The IQ-1S administration regimen induced decreases in systolic blood pressure, mean arterial blood pressure, total peripheral resistance, blood viscosity, hematocrit, myocardial cell cross-sectional area, and aortic wall thickness in SHRs vs untreated SHRs. There were no significant differences in systolic blood pressure values between the control and experimental groups of WKY rats during the treatment period. A concentration-dependent decrease in the tone of carotid arterial rings isolated from SHRs was observed after JNK inhibitor application in vitro. Application of the JNK inhibitor diminished endothelin-1 secretion by human umbilical vein endothelial cells in vitro. The main mechanisms of the antihypertensive effect of IQ-1S included the attenuation of blood viscosity due to decreased hematocrit, a vasodilatory effect on arterial smooth muscle cells, and a decrease in endothelin-1 production by endothelial cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hollander W. Role of hypertension in atherosclerosis and cardiovascular disease. Am J Cardiol. 1976;38:786–800.
Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 2014;7:re3.
Cogolludo A, Pérez-Vizcaíno F, Tamargo J. New insights in the pharmacological therapy of arterial hypertension. Curr Opin Nephrol Hypertens. 2005;14:423–7.
Tassi E, Lai EY, Li L, Solis G, Chen Y, Kietzman WE, et al. Blood pressure control by a secreted FGFBP1 (fibroblast growth factor-binding protein). Hypertension. 2018;71:160–7.
Sadochima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–84.
Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, et al. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–8.
Sugden PH, Clerk A. ‘Stress-responsive’ mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. 1998;83:345–52.
Yano M, Kim S, Izumi Y, Yamamaka S, Iwao H. Differential activation of cardiac c-jun amino-terminal kinase and extracellular signal-regulated kinase in angiotensin II-mediated hypertension. Circ Res. 1998;83:752–60.
Choukroun G, Hajjar R, Kyriakis JM, Bonventre JV, Rosenzweig A, Force T. Role of the stress-activated protein kinases in endothelininduced cardiomyocyte hypertrophy. J Clin Investig. 1998;102:1311–20.
Izumi Y, Kim S, Zhan Y, Namba M, Yasumoto H, Iwao H. Important role of angiotensin II-mediated c-Jun NH(2)-terminal kinase activation in cardiac hypertrophy in hypertensive rats. Hypertension. 2000;36:511–6.
Vogel V, Bokemeyer D, Heller J, Kramer HJ. Cardiac hypertrophy in the Prague-hypertensive rat is associated with enhanced JNK2 but not ERK tissue activity. Kidney Blood Press Res. 2001;24:52–56.
Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97.
Liu X, Huang X, Chen L, Zhang Y, Li M, Wang L, et al. Mechanical stretch promotes matrix metalloproteinase-2 and prolyl-4-hydroxylase α1 production in human aortic smooth muscle cells via Akt-p38 MAPK-JNK signaling. Int J Biochem Cell Biol. 2015;62:15–23.
Duguay D, deBlois D. Differential regulation of Akt, caspases and MAP kinases underlies smooth muscle cell apoptosis during aortic remodelling in SHR treated with amlodipine. Br J Pharm. 2007;151:1315–23.
Zhong JC, Ye JY, Jin HY, Yu X, Yu HM, Zhu DL, et al. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept. 2011;166:90–97.
Reis RI, Nogueira MD, Campanha-Rodrigues AL, Pereira LM, Andrade MCC, Parreiras-E-Silva LT, et al. The binding of Captopril to Angiotensin-I converting enzyme triggers signaling pathways activation. Am J Physiol Cell Physiol. 2018;315:C367–C379.
Li H, Liu X, Zhang L, Li X. LncRNA BANCR facilitates vascular smooth muscle cell proliferation and migration through JNK pathway. Oncotarget. 2017;8:114568–75.
Ok SH, Jeong YS, Kim JG, Lee SM, Sung HJ, Kim HJ, et al. c-Jun NH2-terminal kinase contributes to dexmedetomidine-induced contraction in isolated rat aortic smooth muscle. Yonsei Med J. 2011;52:420–8.
Knight RJ, Buxton DB. Stimulation of c-Jun kinase and mitogen-activated protein kinase by ischemia and reperfusion in the perfused rat heart. Biochem Biophys Res Commun. 1996;218:83–88.
Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–36.
Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591–8.
Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–95.
Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–70. PubMed PMCID: PMC450211
Waetzig V, Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharm Sci. 2005;26:455–61.
Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–8.
Shvedova M, Anfinogenova Y, Atochina-Vasserman EN, Schepetkin IA, Atochin DN. c-Jun N-terminal kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front Pharm. 2018;9:715. eCollection 2018
Schepetkin IA, Kirpotina LN, Khlebnikov AI, Hanks TS, Kochetkova I, Pascual DW, et al. Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors. Mol Pharm. 2012;81:832–45.
Schepetkin IA, Kirpotina LN, Hammaker D, Kochetkova I, Khlebnikov AI, Lyakhov SA, et al. Anti-inflammatory effects and joint protection in collagen-induced arthritis after treatment with IQ-1S, a selective c-Jun N-terminal kinase inhibitor. J Pharm Exp Ther. 2015;353:505–16.
Pearson BD. Indenoquinolines. III. Derivatives of 11H-Indeno-[1,2-b]quinoxaline and related indenoquinolines. J Org Chem. 1962;27:1674–8.
Guide for the Care and Use of Laboratory Animals. National Research Council (US). Committee for the update of the guide for the care and use of laboratory animals. 8th ed. Washington (DC), USA: National Academies Press; 2011.
Lu Q, Qiu TQ, Yang H. Ligustilide inhibits vascular smooth muscle cells proliferation. Eur J Pharm. 2006;542:136–40.
Dornas WC, Silva ME. Animal models for the study of arterial hypertension. J Biosci. 2011;36:731–7.
Zicha J, Kunes J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev. 1999;79:1227–82.
Gómez-Roso M, Montero MJ, Carrón R, Sevilla MA. Cardiovascular changes in spontaneously hypertensive rats are improved by chronic treatment with zofenopril. Br J Pharmacol. 2009;158:1911–21.
Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Exp Rev Cardiovasc Ther. 2018;16:879–87.
Foëx P. Hypertension: pathophysiology and treatment. Cont Educ Anaesth Crit Care Pain. 2004;4:71–75.
Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–73.
Damatto RL, Lima AR, Martinez PF, Cezar MD, Okoshi K, Okoshi MP. Myocardial myostatin in spontaneously hypertensive rats with heart failure. Int J Cardiol. 2016;215:384–7.
Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem. 1998;273:5423–6.
Wu Y, Qian Z, Fu S, Yue Y, Li Y, Sun R, et al. Icariside II improves left ventricular remodeling in spontaneously hypertensive rats by inhibiting the ASK1-JNK/p38 signaling pathway. Eur J Pharm. 2018;819:68–79.
Yao HL, Gao FH, Li ZZ, Wu HX, Xu MD, Zhang Z, et al. Monocyte chemoattractant protein-1 mediates angiotensin II-induced vascular smooth muscle cell proliferation via SAPK/JNK and ERK1/2. Mol Cell Biochem. 2012;366:355–62.
Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–9.
Kim S, Iwao H. Stress and vascular responses: mitogen-activated protein kinases and activator protein-1 as promising therapeutic targets of vascular remodeling. J Pharm Sci. 2003;91:177–81.
Kim S, Murakami T, Izumi Y, Yano M, Miura K, Yamanaka S, et al. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activities are continuously and differentially increased in aorta of hypertensive rats. Biochem Biophys Res Commun. 1997;236:199–204.
Zheng Y, Song HJ, Kim CH, Kim HS, Kim EG, Sachinidis A, et al. Inhibitory effect of epigallocatechin 3-O-gallate on vascular smooth muscle cell hypertrophy induced by angiotensin II. J Cardiovasc Pharm. 2004;43:200–8.
Nagayama K, Kyotani Y, Zhao J, Ito S, Ozawa K, Bolstad FA, et al. Exendin-4 prevents vascular smooth muscle cell proliferation and migration by angiotensin II via the inhibition of ERK1/2 and JNK signaling pathways. PLoS One. 2015;10:e0137960.
Baskurt OK, Yalcin O, Meiselman HJ. Hemorheology and vascular control mechanisms. Clin Hemorheol Microcirc. 2004;30:169–78. PubMed PMID: 15258340
Chien S. Blood rheology in myocardial infarction and hypertension. Biorheology. 1986;23:633–53. PubMed PMID: 3307943
Sloop G, Holsworth RE, Weidman JJ, St Cyr JA. The role of chronic hyperviscosity in vascular disease. Ther Adv Cardiovasc Dis. 2015;9:19–25.
Lee YR, Lee CK, Park HJ, Kim H, Kim J, Kim J, et al. c-Jun N-terminal kinase contributes to norepinephrine-induced contraction through phosphorylation of caldesmon in rat aortic smooth muscle. J Pharm Sci. 2006;100:119–25.
Plotnikov MB, Chernysheva GA, Aliev OI, Smol’iakova VI, Fomina TI, Osipenko AN, et al. Protective effects of a new c-Jun N-terminal kinase inhibitor in the model of global cerebral ischemia in rats. Molecules. 2019;24:E1722.
Shreenivas S, Oparil S. The role of endothelin-1 in human hypertension. Clin Hemorheol Microcirc. 2007;37:157–78. PubMed PMID: 17641406
Sudano I, Roas S, Noll G. Vascular abnormalities in essential hypertension. Curr Pharm Des. 2011;17:3039–44.
McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in hypertension: the role of superoxide anion. Hypertension. 1999;34:539–45.
Jacobsen JC, Hornbech MS, Holstein-Rathlou NH. Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus. 2011;1:117–31.
Atochin DN, Schepetkin IA, Khlebnikov AI, Seledtsov VI, Swanson H, Quinn MT, et al. A novel dual NO-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci Lett. 2016;618:45–49.
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M.Task Force Members et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357.
Ciuffetti G, Pasqualini L, Pirro M, Lombardini R, De Sio M, Schillaci G, et al. Blood rheology in men with essential hypertension and capillary rarefaction. J Hum Hypertens. 2002;16:533–7.
Meiselman HJ, Baskurt OK. Hemorheology and hemodynamics: dove andare? Clin Hemorheol Microcirc. 2006;35:37–43. PubMed PMID: 16899904
Letcher RL, Chien S, Pickering TG, Sealey JE, Laragh JH. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role fibrinogen and concentration. Am J Med. 1981;70:1195–202.
Ajmani RS. Hypertension and hemorheology. Clin Hemorheol Microcirc. 1997;17:397–420. PubMed PMID: 9502525
Linde T, Sandhagen B, Hagg A Mörlin C, Danielson BG. Decreased blood viscosity and serum levels of erythropoietin after anti-hypertensive treatment with amlodipine or metoprolol: results of a cross-over study. J Hum Hypertens. 1996;10:199–205. PubMed PMID: 8733040
Cicco G, Carbonara MC, Stingi GD, Pirrelli A. Cytosolic calcium and hemorheological patterns during arterial hypertension. Clin Hemorheol Microcirc. 2001;24:25–31. PubMed PMID: 11345231
Muravyov AV, Meiselman JH, Yakusevich VV, Zamishlayev AV. Effects of antihypertensive therapy on hemorheological profiles in female hypertensive patients with initially low or high whole blood viscosity. Clin Hemorheol Microcirc. 2002;26:125–35. PubMed PMID: 12082261
Mchedlishvili G, Tsinamdzvrihvili B, Momtselidze N, Beritashvili N. Kinetics of beneficial effect of pentoxifylline on persistent forms of arterial hypertension. Clin Hemorheol Microcirc. 1998;18:285–90. PubMed PMID: 9741669
Cinar Y, Demir G, Pac M, Cinar AB. Effect of hematocrit on blood pressure via hyperviscosity. Am J Hypertens. 1999;12:739–43.
Acknowledgements
This research was supported, in part, by the Tomsk Polytechnic University Competitiveness Enhancement Program. The blood pressure investigation was supported by Russian Science Foundation grant No. 17-15-01111.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Plotnikov, M.B., Aliev, O.I., Shamanaev, A.Y. et al. Antihypertensive activity of a new c-Jun N-terminal kinase inhibitor in spontaneously hypertensive rats. Hypertens Res 43, 1068–1078 (2020). https://doi.org/10.1038/s41440-020-0446-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0446-9