Abstract
Increasing evidence suggests a positive association between the serum uric acid (SUA) level and incident hypertension. However, the association has been inconsistent based on age, sex, body mass index, and lipid profiles. Thus, we investigated whether there is an interaction between SUA and other risk factors on incident hypertension in the Korean general population. In this study, 808 participants aged 40–79 years were included. They were free of hypertension and major cardiovascular disease at baseline. Incident hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or use of antihypertensive medication. To investigate whether the association between SUA and incident hypertension is modified by other risk factors for hypertension, a generalized linear model and Z test were used. During the mean follow-up of 3.3 years, 11.5% of men and 10.7% of women developed hypertension. The association between SUA and incident hypertension was inconsistent according to participant age (p for interaction = 0.009). The association between SUA level and incident hypertension was positively significant among people aged < 55 years (relative risk 1.74 per 1.0 mg/dL of SUA; p = 0.002), but there was no significant association among people aged ≥ 55 years (p = 0.894). In a secondary analysis, the SUA level was not associated with an increase in SBP, but positively associated with DBP. We observed an age-differential association between SUA level and incident hypertension among Koreans. An increased SUA level can be a trigger for hypertension through early vascular changes in the middle-aged population.
Similar content being viewed by others
Introduction
Hypertension, the most important modifiable risk factor [1, 2], is the leading cause of death and a major health burden worldwide [3]. Hypertension was the top risk factor ranked by number of disability-adjusted life-years attributable to stroke for both sexes in a previous study [3]. It is well known that the reduction of highly or moderately elevated blood pressure (BP) levels results in a decrease in stroke and myocardial infarction rates [4, 5]. Every 10 mmHg increase in systolic blood pressure (SBP) or diastolic blood pressure (DBP) is estimated to double the risk of death from ischemic heart disease and stroke [6]. The prevalence of hypertension was estimated as ~ 35.1% for males and 29.1% for females among Korean adults aged 30 years and above in 2015 [7]. Hypertension is an especially common disease among the Korean elderly population aged 65 years or older, and its prevalence increased between 2007 and 2011 from 49.3% to 58.4% in men and from 61.8% to 68.9% in women [8]. The number of people who visited the hospital for chronic diseases in 2015 was 14.39 million in Korea, with the highest number of them for hypertension (5.71 million) [9]. To reduce the overall burden of disease, the prevention of hypertension is crucial.
Serum uric acid (SUA) is the metabolic end product of purines in humans and is known to be a risk factor of hypertension [10,11,12,13]. SUA is positively related not only to the risk of hypertension but also to the risk of atherosclerosis, cardiovascular diseases and metabolic syndrome in previous studies [14,15,16]. Previous studies have also reported that BP is lowered by uric acid-lowering drugs [17,18,19]. The possible linking mechanisms between elevated SUA levels and BP are renal and metabolic abnormalities [16, 20,21,22], systemic inflammation [23] and insulin resistance [24]. However, the associations between SUA and incident hypertension reported in previous studies were inconsistent according to age, sex, lipid profiles, and adiposity level [13, 25, 26]. However, these previous studies that investigate modifiers of the association between SUA and incident hypertension had limitations, such as cross-sectional study design, specific age, or sex. In addition, only limited data are available on the interaction of SUA and other factors for hypertension in the general population. Thus, we investigated whether there is an independent association between SUA and incident hypertension, and whether the association is modified by other risk factors for hypertension among the general Korean population aged 40–79 years.
Methods
Study population
This study analyzed data from the Korean Genome Epidemiology Study (KoGES)-Kangwha, which was a rural, community-based prospective cohort. A total of 4899 people aged > 40 years were enrolled between 2006 and 2011. The participants were living in Kangwha Island, Incheon, South Korea. Eligible participants were asked to participate in the study through on-site invitation, mailed letters, telephone calls, media campaign, or community leader-mediated conferences. After baseline examination, participants underwent a follow-up examination more than once between 2009 and 2013. Detailed recruitment methods for the KoGES-Kangwha study have been reported elsewhere [27, 28]. For this study, we analyzed baseline and the follow-up data of eligible participants (n = 4210) who had information for SUA levels between 2008 and 2013. We excluded individuals who had a history of stroke, angina pectoris or myocardial infarction at baseline (n = 178). We also excluded individuals with high BP (SBP ≥ 140 or DBP ≥ 90 mmHg (n = 458)) and/or taking antihypertensive drugs (n = 655) at baseline. After further excluding individuals who had no measurement of SUA (n = 1192), were missing key covariates (n = 1) or were outliers (n = 11), the final sample was 808 individuals (314 men and 494 women). Incident hypertension was defined by SBP ≥ 140 or DBP ≥ 90 mmHg at follow-up health examinations or a self-report of receiving treatment for high BP and/or a physician’s diagnosis of hypertension during the follow-up period. All participants provided written informed consent, and the study protocol was approved by the Institutional Review Board of Yonsei University Graduate School of Public Health (2-1040939-AB-N-01-2016-403).
Questionnaire data
All participants had an individual interview using standardized questionnaires to obtain information about their demographics, medical history, and health-related lifestyle. All interviewers were trained and performed questionnaire surveys according to a prescribed procedure. Smoking status was categorized as current smoking group and current nonsmoking group (ex-smokers and non-smokers). Alcohol intake was also classified into two groups: current alcohol drinking or current non-drinking (past alcohol drinking and never drinking).
Anthropometrics
Participants were required to refrain from smoking or ingesting caffeine for 8 hours preceding the health examination. Standing height was measured to the ~ 0.1 cm using a stadiometer (SECA763, SECA GMBH, Germany), and body weight was measured to the ~ 0.1 kg using a digital scale (GL-60000-20, Seoul, Korea) with participants wearing underwear and examination gowns. Body mass index (BMI) was calculated as an individual’s body weight in kilograms divided by their height in meters squared (kg/m2). Resting SBP and DBP were measured at least twice using an oscilloscopic sphygmomanometer (Dinamap 1846 SX/P; GE Healthcare, USA). Prior to each measurement, all participants had rested for at least 5 min in a seated position, and the cuff size was adapted to their right upper arm circumference. If the first and second measurements differed by ≥ 10 mmHg, additional measurements were performed, and the average of the last two measurements was used in this analysis. Hypertension was defined as average SBP ≥ 140 mmHg or average DBP ≥ 90 mmHg or current BP medication use.
Laboratory assays
After at least 8 hours of fasting, blood samples were collected from the antecubital vein in the morning. Fasting blood glucose levels were determined by a colorimetry method (ADVIA 1800; Siemens Medical Solutions), and fasting insulin levels were determined by an immunoradiometric assay (SR-300; Stratec, Birkenfeld, Germany). The homeostasis model assessment of insulin resistance (HOMA-IR) was used to evaluate insulin resistance: HOMA-IR = fasting glucose (mg/dL) × fasting insulin (μI U/mL)/405 [29]. Enzymatic methods were used to measure total cholesterol, high-density cholesterol (HDL), triglyceride, and total protein levels (ADVIA 1800; Siemens Medical Solutions, Pleasanton, CA, USA). Low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald formula [30]. Blood urea creatinine was measured by colorimetric methods using automatic analyzers (ADVIA 1650, Bayer Corp, USA). The glomerular filtration rate (GFR) was calculated by the Cockcroft and Gault formula [31]. C-reactive protein (CRP) was measured by a turbidimetric immunoassay assay (ADVIA 1800; DenKa Seiken, Japan).
Statistical analysis
Gender differences in general characteristics were analyzed using the independent t test and the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables. Fasting glucose level, insulin, triglycerides, HOMA-IR, and CRP were log-transformed for parametric testing owing to the right-skewed distribution. To evaluate the independent determinants of incident hypertension, multiple regression analyses were performed. Potential confounders were selected by the results of previous studies and multiple regression analysis using a backward selection method. We also performed goodness-of-fit tests [32].
To access the longitudinal associations between SUA and BP, we used serial generalized linear models with a Poisson distribution using robust variance estimator [32, 33]: model 1 was adjusted for sex (only in the pooled analysis), age and BMI; model 2 was additionally adjusted for SBP and DBP; and model 3 was additionally adjusted for HDL cholesterol and creatinine. This was done when the proportion of the outcome was > 10%, in which case odds ratios would provide biased estimates of associations [34].
To examine the consistency of the observed association between SUA and hypertension, we performed subgroup analyses of participants according to age ( < 55, ≥ 55 years), BMI ( < 25, ≥ 25 kg/m2), triglycerides ( < 150, ≥ 150 mg/dL), HDL cholesterol (40 ≤ , > 40 mg/dL), and LDL cholesterol (130 ≤ , > 130 mg/dL), which are known risk factors for incident hypertension in previous studies. The p value for interactions between SUA and other risk factors on the incident hypertension were calculated by a Z test. In addition, restricted cubic spline was used to investigate the possibility of non-linearity association between SUA and incident hypertension [35]. In this method, we selected five SUA values as knots based on SUA percentiles, tested the linear and non-linear associations between knots using a cubic function, and presented the smoothly integrated graph. Because the restricted cubic spline could be affected by outliers, we excluded values lower than the 1st percentile and greater than the 99th percentile. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA), and statistical significance was defined as a two-sided p value of < 0.05.
Results
Baseline characteristics of the study population are presented in Table 1. The mean age of the study population was 57.1 years in 314 men and 54.3 years in 494 women. The mean level of SUA was 5.7 mg/dL in men and 4.4 mg/dL in women. Age, SBP, DBP, fasting glucose, triglycerides, creatinine, GFR, SUA, smoking, and alcohol intake were significantly higher in men than in women. However, fasting insulin level, total cholesterol, HDL cholesterol, and LDL cholesterol were higher in women than in men. During the follow-up period (mean 3.3 years), 36 men (11.5%), and 53 women (10.7%) developed hypertension.
Table 2 shows the prospective association between SUA levels and incident hypertension using a generalized linear model. The unadjusted relative risk for incident hypertension in total participants was 1.18 (95% CI 1.01–1.37) per 1.0 mg/dL increase in baseline SUA. When adjusted for sex, age, BMI, SBP, DBP, HDL cholesterol and creatinine, the relative risk was 1.19 (95% CI 0.98–1.44) per 1.0 mg/dL increase in baseline SUA. When stratified according to sex, relative risks were 1.20 (95% CI 0.91–1.57) in men and 1.25 (95% CI 0.93–1.69) in women after adjusting for age, BMI, SBP, DBP, HDL cholesterol, and creatinine.
Figure 1 presents the relative risks for incident hypertension according to SUA levels by restricted cubic spline. The relative risks were adjusted for age, BMI, SBP, DBP, HDL cholesterol, and creatinine. Knots were set at the 5th, 25th, 75th, and 95th percentiles, and the plot was truncated at the 1st and 99th percentiles. The median SUA level was used as the reference. There was no significant association between SUA and incident hypertension (p value for the non-linear relation of 0.075; p value for the linear relation of 0.120).
Relative risks for incident hypertension according to SUA levels by restricted cubic spline. The relative risks were adjusted for age, BMI, SBP, DBP, HDL cholesterol, and creatinine. Knots were set at the 5th, 25th, 75th, and 95th percentiles, and the plot was truncated at the 1st and 99th percentiles. The median to SUA level was used as the reference. There was no significant association between SUA and incident hypertension in total population (p value for the non-linear relation of 0.075; p value for the linear relation of 0.120)
Table 3 shows associations between SUA level and incident hypertension among subgroups categorized by risk factors for hypertension including sex, age, BMI, triglycerides, HDL cholesterol, LDL cholesterol, and fasting glucose. The interaction tests for sex, BMI, triglycerides, HDL cholesterol, LDL cholesterol, and fasting glucose were not significant. However, the interaction test for age was significant (p = 0.009). We found that an association between SUA level and incident hypertension was significant in participants of age < 55 years (relative risk 1.74 per 1 mg/dL increase in SUA, p = 0.002), but the association was not significant in participants of age ≥ 55 years (p = 0.894). When we analyzed people age ≥ 55 years divided into people aged 55–64 years and ≥ 65 years, the associations between SUA and incident hypertension did not change (data not shown).
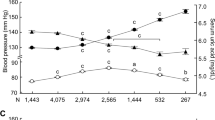
Figure 2 shows the relative risks for incident hypertension according to SUA levels by participant age using restricted cubic spline. In people aged < 55 years, there was a significant linear association between SUA and incident hypertension (p value for the non-linear relation of 0.196; p value for the linear relation of 0.002). However, among people aged ≥ 55 years, there was no significant association between SUA and incident hypertension (p value for the non-linear relation of 0.282; p value for the linear relation of 0.909).
Relative risks for incident hypertension according to serum uric acid levels by participant’s age using restricted cubic spline. The relative risks were adjusted for age, BMI, baseline SBP, baseline DBP, HDL cholesterol, and creatinine. Knots were set at the 5th, 25th, 75th, and 95th percentiles, and the plot was truncated at the 1st and 99th percentiles. The median to SUA level was used as the reference. a In people under the age of 55 years, there was a significant linear association between SUA and incident hypertension (p value for the non-linear relation of 0.196; p value for the linear relation of 0.002). Thus, we show the linear model. b In people over the age of 55 years, there was no significant association between SUA and incident hypertension (p value for the non-linear relation of 0.282; p value for the linear relation of 0.909)
Discussion
We examined whether there is an independent association between SUA and incident hypertension and assessed the interaction between SUA and other risk factors for progression of BP and incident hypertension. We observed an age-differential association between SUA level and incident hypertension. The association between SUA level and incident hypertension was positively significant among people aged < 55 years, but there was no significant association among people aged ≥ 55 years (p for interaction = 0.009).
A previous longitudinal study with a mean follow-up of 5.41 years in a Taiwanese population suggested that the SUA level was an independent predictor of incident hypertension [36]. The Beaver Dam Eye Study, a population-based cohort study of older Americans, observed that increasing quartiles of SUA were associated with 10-year incidence of hypertension independent of smoking, alcohol intake and baseline kidney function [37]. A longitudinal study over 8 years with Americans also reported that higher baseline SBP and lower HDL cholesterol were significant independent predictors for incident hypertension in a multivariate Cox regression model [38].
In a cross-sectional study that investigated the interaction of BP and other risk factors on hypertension, the interaction between SUA and triglyceride was significant for SBP, but not for DBP, after adjusting for sex and age [26]. A higher SUA level was significantly associated with prehypertension in a group with triglyceride levels of < 150 mg/dL but not in a group with triglyceride levels of ≥ 150 mg/dL. Another study reported that there was an independent and positive association between serum acid and hypertension, suggesting that HDL cholesterol may modify the association between SUA and hypertension [25]. In this previous study, the association between SUA and hypertension was most prominent in those with the highest quintiles of HDL cholesterol. On the other hand, there was an independent association between baseline lipids (total cholesterol, HDL cholesterol and total cholesterol/HDL cholesterol ratio) and hypertension in the Physicians’ Health Study among men without diabetes and obesity [39]. This previous study suggested that dyslipidemia may lead to the subsequent development of hypertension. The Women’s Health Study also reported that dyslipidemia was independently associated with the subsequent development of hypertension among healthy women [40]. The Tehran Lipid and Glucose Study also suggested that dyslipidemia measured by serum triglycerides and the triglyceride/HDL cholesterol ratio may be useful in identification of women at risk of hypertension [41]. However, the interactions of SUA with lipid profiles on BP were not significant in the current study.
It is well known that the SUA level is higher in men than in women. Some previous studies reported that sex is an important factor in interrelationships among age, hyperuricemia, and metabolic syndrome, including the component of hypertension [42, 43]. Also, a previous study reported that the menopausal status of women was an important factor of the hyperuricemia and metabolic syndrome [44, 45]. Different roles of xanthine oxidase and oxidative stress, and estrogen levels according to sex can cause a sex-differential association between SUA and incident hypertension [46, 47]. Thus, we analyzed data with a stratification for sex, but sex did not significantly influence the association between SUA and incident hypertension in the current study. Further studies with larger sample sizes are needed to identify the sex-differential association between SUA and incident hypertension.
A longitudinal study with Japanese men aged 18–60 years reported that high a SUA level was associated with future hypertension, and this association was stronger in participants aged 40–60 years than that of those aged 18–40 years (p for interaction = 0.035) [13]. Our study included people aged 40–79 years, and we observed that the association between SUA level and incident hypertension was more prominent in participants aged 40–55 years than in those aged ≥ 55 years. Considering these results together, an elevated SUA level can be a trigger for hypertension, especially in the middle-aged population.
The mechanism linking the association between increased SUA level and incident hypertension is not completely understood. Previous studies reported that oxidative stress, inflammation, nitric oxide production impairment, vascular endothelial dysfunction, vascular smooth muscle proliferation, and renin–angiotensin system enhancement were mechanisms for incident hypertension by hyperuricemia [48,49,50,51,52]. Crystallization of uric acid itself has also been reported to cause inflammation, gouty kidney, and urinary tract, and progression to renal failure [53, 54]. High SUA levels may lead to decreasing endothelial nitric oxide, which is well known as a mediator of insulin action and increases blood flow to skeletal muscle and enhances glucose uptake [48]. High SUA can cause renal vasoconstriction and alters the proliferation/migration of endothelial and vascular smooth muscle cells through inhibition of nitric oxide and stimulation of the renin–angiotensin system, which may then lead to endothelial dysfunction [49, 50, 55, 56]. Thus, hyperuricemia may lead to raised BP. In addition, SUA is an indicator of systematic inflammation [57] and is associated with cardiovascular risk factors such as insulin resistance [58], BMI, total cholesterol, HDL cholesterol, triglycerides, and fasting glucose [58,59,60].
In additional analyses, we considered blood lipids as covariates for incident hypertension, although these variables, including total cholesterol, LDL cholesterol, and triglycerides, were not influential in a generalized linear model (data not shown). Dyslipidemia may lead to impairment in endothelial function and result in defective vasoregulation [61], increasing arterial stiffness [62], decreasing compliance, and renal microvascular disease. High triglyceride levels may cause endothelial dysfunction [63], loss of vasomotor reactivity [64], and arterial stiffness [65]. A high triglyceride level is significantly associated with insulin resistance [49], which may lead to incident hypertension by promoting renal tubular sodium reabsorption, stimulating sympathetic nervous system reactivity and the renin–angiotensin system [25]. Both SUA and lipids can cause incident hypertension through endothelial dysfunction and systematic inflammation [25, 26, 41, 66, 67]. However, according to our analysis, blood lipids at baseline were not independent predictors of future hypertension, but SUA, BMI, SBP, and DBP were independent predictors for future hypertension when fully adjusted (data not shown). The results suggested that such unfavorable pathophysiological processes induced by SUA might be greater than those of blood lipids.
In our study, the association between SUA and incident hypertension was inconsistent between two subgroups categorized by age. We cannot explain exactly how age modifies the effects of uric acid on the development of hypertension.
One possible explanation can be pathophysiological changes with aging. If SUA can cause premature vascular degeneration, SUA could not affect elderly people whose pathophysiological changes, including premature vascular degeneration and endothelial dysfunction, may have already occurred with aging. Similar to a well-known process of progression in BP [68], increased SUA level can be a trigger for hypertension through early vascular change in the middle-aged population but not among people aged ≥ 55 years who already had some vascular degeneration. A previous study also suggested that the SUA level might play a role in the early pathogenesis of primary hypertension [69, 70]. Furthermore, a previous study reported that the strength of the relationship between uric acid and hypertension decreases with increasing patient age and duration of hypertension, suggesting that SUA may be most important in younger people with early-onset hypertension [71].
Another possible explanation is that SUA is associated with an increase in DBP rather than SBP. Previous studies have reported that the prevalence of isolated systolic hypertension (SBP ≥ 160 and DBP < 90 mmHg) rises with age [72,73,74,75]. SBP increases with age, at least until over 80 years of age, but DBP only rises until 50–60 years of age and thereafter either levels off or slightly decreases. In a study with Korean adults, mean SBP increased progressively across the entire age range [76]. However, mean DBP increased slightly before the age of 55 years and then plateaued or decreased. In the 2011 Korean National Health and Nutrition Examination Survey, SBP steadily increased with age until more than 60 years, whereas DBP decreased, resulting in an increase in the pulse pressure [77]. In our secondary analysis, the SUA level was not associated with an increase in SBP but was positively associated with an increase in DBP (Supplementary Table 1). Considering these results, higher SUA is associated with an increase in DBP rather than SBP, and a higher SUA level could not affect participants over the age of 55 years because DBP levels get closer to maximum at the age of 55 years. The age-differential association between SUA and incident hypertension in our study can be partly explained by isolated systolic hypertension, which predominates after the age of 50 years as SBP continues to rise and DBP tends to fall. Further studies are needed to clarify the interrelationship between SUA and age for incident hypertension and which group is most vulnerable to incident hypertension.
The current study has some limitations. First, it may not be appropriate to generalize it to another ethnic group because this study was conducted among Korean adults from a single rural community. Second, we could not take into account day-to-day variation of BP because BP level was decided in a single visit, although we conducted BP measurement multiple times. These may have led to a misclassification of incident hypertension. However, the effects of non-differential misclassification would have resulted in a bias toward the null. Third, we did not consider medications for diabetes and dyslipidemia as covariates. Fasting glucose levels and blood lipids can be influenced by these medications. Fourth, although we took a large number of potential confounders into consideration, the possibility remains that unmeasured factors such as overall nutrition or specific dietary nutrient intake could account for the association of SUA with incident hypertension. However, when we analyzed data with additional adjustment for some blood test results, including albumin and protein that reflect the overall nutritional status, our main finding regarding the age-differential association between SUA and incident hypertension did not change. When we performed subgroup analysis including only people with BMI ≥ 18.5 kg/m2 to consider the effect of undernutrition, the main result was also not changed (data not shown).
In conclusion, we observed an age-differential association between SUA level and incident hypertension among a community-dwelling healthy Korean population. Among adults aged 40–79 years, a high SUA level was significantly associated with incident hypertension only in adults under the age of 55 years. To prevent future hypertension and cardiovascular disease effectively, active intervention to avoid increasing the SUA level may be required, especially in the middle-aged healthy population.
References
Lee SW, Kim HC, Lee HS, Suh I. Thirty-year trends in mortality from cardiovascular diseases in Korea. Korean Circ J. 2015;45:202–9.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62.
Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–24.
Neaton JD, Grimm RH, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, et al. Treatment of mild hypertension study: final results. JAMA. 1993;270:713–24.
Dollery C. Hypertension trial results: consensus and conflicts. J Hum Hypertens. 1995;9:403–8.
Lewington S. Prospective studies collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies (vol 360, pg 1903, 2002). Lancet. 2003;361:1060–1060.
Korea Health Statistics 2015: Korea National Health and Nutrition Examination Survey (KNHANES VI). Ministry of Health and Welfare. [Internet]. [Accessed on Oct 2, 2017]. Available from: http://kostat.go.kr/portal/korea/.
Shin J, Park J, Kim K, Kim J, Yang D, Pyun W, et al. Korean Society of Hypertension guidelines for the management of hypertension. Part I-epidemiology and diagnosis of hypertension. Clin Hypertens. 2015;21:1.
Medical Service Usage Statistics by Region. 2015. National Health Insurance Corporation. [Internet]. Available from: http://www.nhis.or.kr/bbs7/boards/B0075. Accessed 9 Apr 2017.
Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med. 2009;169:155–62.
Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta‐analysis. Arthritis Care Res. 2011;63:102–10.
Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, et al. Uric acid and the development of hypertension the normative aging study. Hypertension. 2006;48:1031–6.
Yokoi Y, Kondo T, Okumura N, Shimokata K, Osugi S, Maeda K, et al. Serum uric acid as a predictor of future hypertension: Stratified analysis based on body mass index and age. Prev Med. 2016;90:201–6.
Ishizaka N, Ishizaka Y, Toda E-I, Nagai R, Yamakado M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25:1038–44.
Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke the Rotterdam study. Stroke. 2006;37:1503–7.
Nagano S, Takahashi M, Miyai N, Oka M, Utsumi M, Shiba M, et al. Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive subjects. Hypertens Res. 2017;40:620.
Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a ystematic review and meta‐analysis. J Clin Hypertens. 2013;15:435–42.
Feig DI. The role of uric acid in the pathogenesis of hypertension in the young. J Clin Hypertens. 2012;14:346–52.
Ohta Y, Ishizuka A, Arima H, Hayashi S, Iwashima Y, Kishida M, et al. Effective uric acid-lowering treatment for hypertensive patients with hyperuricemia. Hypertens Res. 2017;40:259.
Facchini F, Chen Y-DI, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–11.
Galvan AQ, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol-Endocrinol Metab. 1995;268:E1–E5.
Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med. 1980;93:817–21.
Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS ONE. 2011;6:e19901.
Taniguchi Y, Hayashi T, Tsumura K, Endo G, Fujii S, Okada K. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. J Hypertens. 2001;19:1209–15.
Teng F, Zhu R, Zou C, Xue Y, Yang M, Song H, et al. Interaction between serum uric acid and triglycerides in relation to blood pressure. J Hum Hypertens. 2011;25:686–91.
Kawamoto R, Tabara Y, Kohara K, Kusunoki T, Abe M, Miki T. Interaction between serum uric acid and triglycerides in relation to prehypertension in community-dwelling Japanese adults. Clin Exp Hypertens. 2014;36:64–69.
Kim Y, Han B-G, group K. Cohort profile: the Korean genome and epidemiology study (KoGES) consortium. Int J Epidemiol. 2016;46:e20–e20.
Cho HM, Kim HC, Lee J-M, Oh SM, Choi DP, Suh I. The association between serum albumin levels and metabolic syndrome in a rural population of Korea. J Prev Med Public Health. 2012;45:98.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:1.
Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6.
Deddens J, Petersen MR, Endahl L. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol. 1998;27:91–95.
Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Prog Biomed. 1997;54:201–8.
Yang T, Chu C-H, Bai C-H, You S-L, Chou Y-C, Hwang L-C, et al. Uric acid concentration as a risk marker for blood pressure progression and incident hypertension: a Chinese cohort study. Metabolism. 2012;61:1747–55.
Shankar A, Klein R, Klein B, Nieto F. The association between serum uric acid level and long-term incidence of hypertension: population-based cohort study. J Hum Hypertens. 2006;20:937–45.
Wildman RP, Sutton‐Tyrrell K, Newman AB, Bostom A, Brockwell S, Kuller LH. Lipoprotein levels are associated with incident hypertension in older adults. J Am Geriatr Soc. 2004;52:916–21.
Halperin RO, Sesso HD, Ma J, Buring JE, Stampfer MJ, Gaziano JM. Dyslipidemia and the risk of incident hypertension in men. Hypertension. 2006;47:45–50.
Sesso HD, Buring JE, Chown MJ, Ridker PM, Gaziano JM. A prospective study of plasma lipid levels and hypertension in women. Arch Intern Med. 2005;165:2420–7.
Tohidi M, Hatami M, Hadaegh F, Azizi F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J Hum Hypertens. 2012;26:525–32.
Chiou W-K, Wang M-H, Huang D-H, Chiu H-T, Lee Y-J, Lin J-D. The relationship between serum uric acid level and metabolic syndrome: differences by sex and age in Taiwanese. J Epidemiol. 2010;20:219–24.
Chien K-L, Hsu H-C, Sung F-C, Su T-C, Chen M-F, Lee Y-T. Hyperuricemia as a risk factor on cardiovascular events in Taiwan: the Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis. 2005;183:147–55.
Un J-D, Chiou W-K, Chang H-Y, Liu F-H, Weng H-F, Liu T-H. Association of hematological factors with components of the metabolic syndrome in older and younger adults. Aging Clin Exp Res. 2006;18:477–84.
Lai S, Tan C, Ng K. Epidemiology of hyperuricemia in the elderly. Yale J Biol Med. 2001;74:151.
Nicholls A, Snaith M, Scott J. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1:449–51.
Simon JA, Lin F, Vittinghoff E, Bittner V. The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: the Heart and Estrogen–Progestin Replacement Study (HERS). Ann Epidemiol. 2006;16:138–45.
Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–86.
Mazzali M, Hughes J, Kim Y-G, Jefferson JA, Kang D-H, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–6.
Kang D-H, Park S-K, Lee I-K, Johnson RJ. Uric acid–induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–62.
Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin–angiotensin system. J Hypertens. 2008;26:269–75.
Kono H, Chen C-J, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–49.
Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239–47.
Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol 2008;19:1204–11.
Lai S-W, Ng K-C. Which anthropometric indices best predict metabolic disorders in Taiwan? South Med J. 2004;97:578–83.
Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–42.
Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, et al. Uric acid and survival in chronic heart failure validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–7.
Vuorinen-Markkola H, Yki-Järvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab. 1994;78:25–29.
Cigolini M, Targher G, Tonoli M, Manara F, Muggeo M, De Sandre G. Hyperuricaemia: relationships to body fat distribution and other components of the insulin resistance syndrome in 38-year-old healthy men and women. Int J Obes Relat Metab Disord. 1995;19:92–96.
Chu N-F, Wang D-J, Liou S-H, Shieh S-M. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur J Epidemiol. 2000;16:13–17.
Selwyn AP, Kinlay S, Libby P, Ganz P. Atherogenic lipids, vascular dysfunction, and clinical signs of ischemic heart disease. Circulation. 1997;95:5–7.
Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–91.
Jagla A, Schrezenmeir J. Postprandial triglycerides and endothelial function. Exp Clin Endocrinol Diabetes. 2001;109:533–47.
Lundman P, Eriksson M, Schenck-Gustafsson K, Karpe F, Tornvall P. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation. 1997;96:3266–8.
Yao W, Zhang H, Zhu Z, Zhou Y, Liang N, Xu D, et al. Genetically elevated levels of circulating triglycerides and brachial–ankle pulse wave velocity in a Chinese population. J Hum Hypertens. 2013;27:265–70.
Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33.
Kawamoto R, Katoh T, Ninomiya D, Kumagi T, Abe M, Kohara K. Synergistic association of changes in serum uric acid and triglycerides with changes in insulin resistance after walking exercise in community-dwelling older women. Endocr Res. 2016;41:116–23.
Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti A. Endothelium, aging, and hypertension. Curr Hypertens Rep. 2006;8:84–89.
Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–52.
Feig DI, Kang D-H, Johnson RJ. Uric acid and cardiovascular risk. New Engl J Med. 2008;359:1811–21.
Brand F, McGee D, Kannel W, Stokes J, Castelli W. Hyperuricemia as a risk factor of coronary heart disease: the Framingham Study. Am J Epidemiol. 1985;121:11–18.
Staessen J, Amery A, Fagard R. Isolated systolic hypertension in the elderly. J Hypertens. 1990;8:393–405.
Chobanian AV. Isolated systolic hypertension in the elderly. New Engl J Med. 2007;357:789–96.
Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease: the Framingham study. Am J Cardiol. 1971;27:335–46.
Staessen J, Bulpitt C, Fagard R, Joossens JV, Lijnen P, Amery A. Four urinary cations and blood pressure. A population study in two Belgian towns. Am J Epidemiol. 1983;117:676–87.
Jo I, Ahn Y, Lee J, Shin KR, Lee HK, Shin C. Prevalence, awareness, treatment, control and risk factors of hypertension in Korea: the Ansan study. J Hypertens. 2001;19:1523–32.
Shin J, Park JB, Kim KI, Kim JH, Yang DH, Pyun WB, et al. 2013 Korean Society of Hypertension guidelines for the management of hypertension: part I-epidemiology and diagnosis of hypertension. Clin Hypertens. 2015;21:1.
Acknowledgements
This study was supported by grants from the Korea Centers for Disease Control and Prevention (2006-347-2400-2440-215, 2008-E71004-00, 2009-E71006-00, 2010-E71003-00, 2011-E71002-00, 2012-E71007-00, 2013-E71008-00) and the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI13C0715).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, S.W., Kim, H.C., Nam, C. et al. Age-differential association between serum uric acid and incident hypertension. Hypertens Res 42, 428–437 (2019). https://doi.org/10.1038/s41440-018-0168-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0168-4
Keywords
This article is cited by
-
Comorbidities of nontuberculous mycobacteria infection in Korean adults: results from the National Health Insurance Service–National Sample Cohort (NHIS–NSC) database
BMC Pulmonary Medicine (2022)
-
Development of a risk prediction score for hypertension incidence using Japanese health checkup data
Hypertension Research (2022)
-
Association of childhood anthropometric measurements and laboratory parameters with high blood pressure in young adults
Hypertension Research (2021)
-
J-shaped curve for the association between serum uric acid levels and the prevalence of blood pressure abnormalities
Hypertension Research (2021)
-
High serum uric acid within the normal range is a useful predictor of hypertension among Japanese community-dwelling elderly women
Clinical Hypertension (2020)