Abstract
Purpose
To assess the clinical performance of an expanded noninvasive prenatal screening (NIPS) test (“NIPS-Plus”) for detection of both aneuploidy and genome-wide microdeletion/microduplication syndromes (MMS).
Methods
A total of 94,085 women with a singleton pregnancy were prospectively enrolled in the study. The cell-free plasma DNA was directly sequenced without intermediate amplification and fetal abnormalities identified using an improved copy-number variation (CNV) calling algorithm.
Results
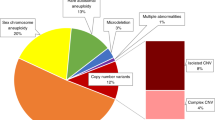
A total of 1128 pregnancies (1.2%) were scored positive for clinically significant fetal chromosome abnormalities. This comprised 965 aneuploidies (1.026%) and 163 (0.174%) MMS. From follow-up tests, the positive predictive values (PPVs) for T21, T18, T13, rare trisomies, and sex chromosome aneuploidies were calculated as 95%, 82%, 46%, 29%, and 47%, respectively. For known MMS (n = 32), PPVs were 93% (DiGeorge), 68% (22q11.22 microduplication), 75% (Prader–Willi/Angleman), and 50% (Cri du Chat). For the remaining genome-wide MMS (n = 88), combined PPVs were 32% (CNVs ≥10 Mb) and 19% (CNVs <10 Mb).
Conclusion
NIPS-Plus yielded high PPVs for common aneuploidies and DiGeorge syndrome, and moderate PPVs for other MMS. Our results present compelling evidence that NIPS-Plus can be used as a first-tier pregnancy screening method to improve detection rates of clinically significant fetal chromosome abnormalities.
Similar content being viewed by others
INTRODUCTION
In the human, chromosome abnormalities such as aneuploidies and segmental imbalances are common and originate from either meiotic nondisjunction errors or mitotic replication errors often in the preimplantation embryo stage.1 Typically, they result in either embryo growth arrest, implantation failure, or early miscarriage during the first trimester.2 However, 1–1.7% of fetuses that remain developmentally competent are affected with chromosome abnormalities,3 and if undetected by prenatal diagnosis, can persist through the second and third trimester and manifest as chromosome disease syndromes at birth. Approximately 15% of the major congenital abnormalities diagnosed before the age of 1 year are caused by chromosomal abnormalities and, of these, 25% result in perinatal deaths.
Karyotyping and microarrays have been the de facto diagnostic methodologies used for confirmatory chromosome testing of prenatal samples obtained by invasive amniocentesis or chorionic villus sampling (CVS)3,4 after indications of increased risk of potentially abnormal fetus development following first trimester screening (FTS) (biochemical and ultrasound screening).4 The discovery of fetal DNA in the cell-free plasma of pregnant women and the development of next-generation sequencing (NGS) based methods enabling detection of fetal chromosome abnormalities have revolutionized reproductive medicine.5 With the widening adoption of noninvasive prenatal screening (NIPS) in the last 7 years, many women with pregnancies classified as either high- or low-risk through traditional screening methods are electing for NIPS with its greater sensitivity and much higher specificity than maternal serum screening for detection of the more common fetal trisomies6,7 as well as the sex chromosomal aneuploidy (SCA) 45,XO.8,9 This change in patient choice has led to a reduction in invasive testing requests by up to 40% (ref. 10).
More recently, further development and expansion of NIPS has focused on additional analysis for microdeletion/microduplication syndromes (MMS) caused by segmental chromosome imbalances.11 Although MMS are relatively rare, collectively they represent a significant group of chromosome diseases,12 accounting for 1–2% of all newborn congenital abnormalities and often resulting in a severe burden for families and society. Deletions of the chromosome 22q11.21 region are associated with DiGeorge syndrome (DGS) and are the most frequently detected MMS, occurring in 1 in 3000–6000 newborns.13 Several clinical research NIPS studies successfully demonstrated proof of concept for cell-free detection of MMS using either deeper sequencing or higher-density single-nucleotide polymorphism (SNP) targeting methodologies.14,15,16
Known biological factors such as low fetal DNA fraction and confined placental mosaicism (CPM),17 which can confound any NIPS results, make reliable and accurate detection of MMS challenging.4,11,18 In reported clinical validation studies, expanded NIPS tests tended to display variable performance for detection of specific MMS, with only low to moderate positive predictive values (PPVs) reported for DGS, Prader–Willi/Angleman syndrome (PWS), cri du chat (CDC), and 1p36 microdeletion (1p36 del) syndrome.19,20,21,22,23 Recent guidelines from the American College of Medical Genetics and Genomics (ACMG),24 following active debate in the field,25,26 resulted in a general consensus that the introduction of expanded NIPS is not appropriate until improved performance is demonstrated in a clinical setting involving a large patient cohort.
To address this issue, we designed and developed an advanced pipeline called NIPS-Plus that utilizes deeper sequencing than traditional NIPS, with combinatorial data analysis algorithms to additionally call genome-wide copy-number variations (CNVs) associated with MMS. Here, we prospectively analyze 94,085 singleton pregnancies using NIPS-Plus and report on its performance for simultaneous detection of both common aneuploidies and MMS.
MATERIALS AND METHODS
Study oversight
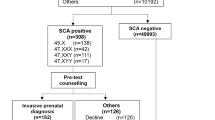
The clinical research study (Fig. 1) was approved by the Ethics Committee of State Key Laboratory of Medical Genetics (number 2013102301). Pregnant women were not offered free prenatal testing as a condition of entering the study and thus there was no undue influence for participation. Enrollment procedures for this study fully complied with all individual hospital and national ethics guidelines. Women were enrolled following existing procedures previously established for NIPS at prenatal diagnosis centers accredited and authorized by the Provincial Health Administration and entered an informed consent process specifically designed for NIPS-Plus including notice of sample type, test method, screening-covered diseases, and limitations and risks. Other conventions such as details of the insurance program provided and a statement of study compliance with hospital guidelines, state laws, and national ethics guidelines, were also included on the consent form. In addition, every woman was provided with the laboratory requisitions and pretest counseling information on the sensitivity, specificity, PPV, and negative predictive value (NPV) of the NIPS-Plus assay for trisomies, SCAs, and CNVs, which was previously derived from a pilot validity and clinical utility study conducted at the State Key Laboratory of Medical Genetics.
First-tier screening pipeline for detection of chromosome disease syndromes. cfDNA cell-free DNA, CNV copy-number variation, GC Guanine and Cytosine content, GD gene database, HMM hidden Markov model, NGS next-generation sequencing, NIPS nonivasive prenatal screening, PCA principal component analysis, PCR polymerase chain reaction, QC quality control.
Qualified professionals at each accredited prenatal center ensured that all the contents of the informed consent form were read and understood before signing. In regard to insurance, all women were registered at ChinaLife under a specific NIPS-Plus insurance scheme covering the standard and expanded test range. The final prospective enrolled study cohort comprised 94,085 Chinese women with singleton pregnancies who were analyzed by NIPS-Plus for clinically significant fetal aneuploidies and MMS.
Patient demographics
The 94,085 patients, recruited to the study between November 2015 and December 2017, were from the general population of reproductive women who had naturally conceived a singleton pregnancy. There were 38,023 (40.41%) high-risk pregnancies where maternal age was ≥35 years (median of 37 years, range 35–53 years), 56,058 (59.58%) low-risk pregnancies where maternal age was <35 years (median of 29 years, range 15–34 years) who opted for the test, and 4 (0.004%) pregnancies with an undisclosed maternal age. Blood samples were generally collected early in the second trimester with a median gestational age (GA) of 17+3 weeks (range 11–39 weeks), excluding 31 cases with an unknown GA and 23 cases with GA less than 11 weeks, which is current standard practice in China. The ENET algorithm27 was used to calculate fetal fraction (FF) for all pregnancies. The redraw rate, based on either low FF (<3% threshold) of the first plasma sample or poor quality blood samples due to hemolysis, was 0.22% and 0.31%, respectively. The median fetal DNA fraction of the final set of tested samples passing quality control (QC) was 10.8% (3.0–47.6%).
Assay optimization for NIPS-Plus
We optimized the molecular techniques applied in this study (Fig. 1), by modifying key steps in our original NIPS pipeline.6,28 In brief, 10 ml blood samples were collected in Streck tubes (Streck, USA) and cell-free DNA (cfDNA) purified from the plasma fraction using the fetal chromosome aneuploidy (T21/T18/T13) test kit developed by Berry Genomics (Beijing, China). Approximately 10 ng of cfDNA was then used without further amplification to construct cfDNA libraries. After quantification, libraries were tag sequenced on the NextSeq CN500 platform (Illumina) to generate approximately 20 M 45-bp reads (37-bp + 8-bp index), which were trimmed to generate 36-bp genomic sequence reads (fastq file format). Raw reads (15–30 M) were mapped to hg19 reference genome using the RUPA algorithm developed by Berry Genomics and then the uniquely mapped reads (10–19 M) were analyzed. Reads were allocated to continuous nonoverlapping 100-kb bins and further filtered to remove bins with abnormal GC content (<30% or >70%) and low coverage.
For accurate CNV calling from NGS data, data variability from region to region and from sample to sample is the predominant source of noise. Increasing the signal-to-noise ratio was therefore the overarching objective in our algorithmic development. Using algorithm advances for detecting CNVs from NGS data,29 we developed a principal component analysis (PCA) based method to address the signal-to-noise ratio issue. Based on a large training data set (N = 2000) obtained from actual NIPS sequencing data with the assumption that all samples were negative for any MMS in fetal genomes, we removed the first ten principal components on each chromosome. We then developed a hidden Markov model (HMM) to detect the CNVs.
Pregnancy management
Pregnant women underwent a routine fetal ultrasound scan at 18 weeks gestation. All suspected NIPS-Plus positive pregnancies were offered free amniocentesis and confirmatory prenatal diagnosis under the NIPS-Plus insurance scheme. Whole chromosome aneuploidies were confirmed by karyotyping and segmental imbalances associated with MMS were confirmed by molecular karyotyping using either SNP arrays or CNV-Seq. The pathogenicities of CNVs detected by NIPS-Plus were evaluated following the ACMG guidelines.24 Only pathogenic or likely pathogenic CNVs were reported to the clinician. Any false negative NIPS-Plus results subsequently identified by either prenatal ultrasound or clinical examination of a newborn had chromosome testing to confirm the presence of any unidentified MMS. All women who carried a fetus suspected of having a confirmed pathogenic or likely pathogenic fetal chromosome anomaly were scheduled for a genetic counseling session to discuss pregnancy management options.
RESULTS
Fetuses identified with probable chromosomal abnormalities
The final study cohort comprised 94,085 prospective Chinese women with a singleton pregnancy analyzed by NIPS-Plus (Fig. 1) for clinically significant fetal aneuploidies and MMS. A total of 1128 fetuses (1.2%) were suspected to have pathogenic or likely pathogenic chromosome anomalies (Table 1). Of these, 965 fetuses (85%) were positive for whole chromosome aneuploidies and all were followed up by amniocentesis and karyotyping. The remaining 163 fetuses (15%) were scored positive for a MMS involving segmental imbalances (CNVs). Of these 120 (74%) were followed up by either CNV-Seq or chromosomal microarray (CMA) on their respective amniocentesis samples (Supplementary Table 1) and were used as the study data set to evaluate the performance of NIPS-Plus for MMS detection.
Fetuses with suspected trisomies and SCAs
Of the 965 NIPS-Plus positive results, there were 526 fetuses at high risk for T21, T18, or T13 (Table 1). T21 (n = 364) was the most common, followed by T18 (n = 123) and T13 (n = 39). Of these, there were 20 pregnancies incorrectly scored as high risk (FPs) for T21, 22 for T18, and 21 for T13, yielding positive predictive values (PPVs) of 95%, 82%, and 46%, respectively. Three cases classified as low risk of T21 and two cases of low risk for T18 were identified by follow-up diagnoses, giving negative predictive values (NPV) of 99.997% and 99.998% respectively. All cases of T13 were correctly identified.
In addition, 37 fetuses were also scored as positive for less common autosomal aneuploidies including trisomies T1 (n = 1), T2 (n = 3), T3 (n = 1), T5 (n = 1), T7 (n = 13), T8 (n = 1), T10 (n = 1), as well as monosomies M13 (n = 4), M18 (n = 6), and M21 (n = 6). Further, 12 fetuses were scored as positive for multiple rare autosomal trisomies (Table 1). Of these 49 cases, 35 were later shown as incorrectly classified following confirmatory testing, yielding a combined PPV for other rare autosomal aneuploidies of 29%.
There were 390 fetuses that scored positive for a sex chromosome aneuploidy (SCA), including 190 (48.7%) with suspected Turner syndrome (45,X), 76 (19.5%) with Klinefelter syndrome (47,XXY), 81 (20.8%) with triple X syndrome (47,XXX), 24 (6.2%) with Jacob syndrome (47,XYY), and 19 (4.8%) with 46,XY (X del) (Table 1). Within this group, 141 of 190 were incorrectly identified as 45,X; 13 of 76 as 47,XXY; 31 of 81 as 47,XXX; 6 of 24 as 47,XYY; and 17 of 19 for 46,XY (X del), yielding individual PPVs of 26%, 83%, 62%, 75%, and 11%, respectively.
Fetuses with suspected MMS
For the 120 pathogenic or likely pathogenic fetal CNVs that were followed up in validation studies (Table 1), there were 32 cases of MMS associated with classical chromosome diseases. This comprised 14 cases at high risk of DGS, 6 cases of 22q microduplication syndrome, 4 cases of PWS, 6 cases of CDC, and 2 cases of 1p36 del syndrome (Fig. 2, selected examples). Of the 14 cases of suspected DGS, there were 13 true positives (TPs) and 1 FP yielding a PPV of 93%. In addition, at the same loci, there were six cases of 22q11.2 microduplications comprising four TPs and two FPs (PPV 67%). For the four suspected PWS cases, there were three TPs and one FP (PPV 75%) and for the six suspected CDC cases, there were three TPs and three FPs (PPV 50%). Lastly, the two cases indicated as 1p36 deletions both proved to be FPs. The remaining 88 of 120 fetal CNVs comprised genome-wide segmental CNVs that were classified as nonsyndromic MMS because no specific syndromes could be identified in any current databases as associated with these changes. Of these, there were 23 TPs and 49 FPs for CNVs ≥10 Mb (PPV 32%) and 3 TPs and 13 FPs for CNVs <10 Mb (PPV 19%). These latter results, while nonsyndromic, are presented for the sake of completeness and as evidence of general utility of the analysis method in finding CNVs throughout the genome.
Confirmatory analysis of microdeletion/microduplication syndromes (MMS) detected by NIPS-Plus. Comparative chromosome plots from NIPS-Plus (left) and CNV-Seq (right) analyses. Selected examples of true positive, false positive, and false negative results for 22q11.2 deletions are shown. Copy-number variations (CNVs) are highlighted by red boxes. cfDNA cell-free DNA.
In nine pregnancies, both a fetal and maternal CNV of equivalent size were codetected (Supplementary Table 2). These included five cases of 22q microduplication, one case each of DGS and CDC, and two cases of nonsyndromic CNVs. Using confirmatory chromosome testing, all maternal CNVs were confirmed whereas only six of the nine fetal CNVs were correctly classified, giving an overall PPV of 67% for correctly calling both a maternal and fetal CNV. Further, in seven pregnancies only maternal CNVs were detected, including a case of DGS and six cases of other nonsyndromic CNVs (Supplementary Table 3). Follow-up testing confirmed all seven maternal CNVs, giving a PPV of 100% for calling only maternal CNVs.
Clinical outcomes of confirmed positive fetuses
All women with true positive NIPS-Plus results were offered genetic counseling to discuss pregnancy management. All women with confirmed T18 and T13 fetuses elected termination of pregnancy (TOP). While the large majority of women diagnosed with either a T21 fetus (66.9%) or a SCA fetus (40.7%) also elected TOP, there was a relatively significant proportion who chose to continue their pregnancies. Elective TOP rates were much higher with pregnancies diagnosed with known MMS, including DGS (92%), PWS (100%), and CDC (100%) (Supplementary Table 1). In contrast, elective TOP rates were much lower for women carrying a fetus with 22q microduplication syndrome (33.3%).
There were ten pregnancies with a pathogenic chromosome anomaly missed by NIPS-Plus (NPV 99.99%) (Table 2). Of the false negative (FN) cases, 3 of 347 cases involved T21 (sensitivity of 99%) and 2 of 103 cases involved T18 (sensitivity of 98%). For the MMS, 2 of 15 cases involved DGS (sensitivity of 87%), 1 of 4 cases involved PWS (sensitivity of 75%), 1 of 4 cases involved CDC (sensitivity of 75%), and 1 of 1 cases involved 1p36 deletion syndrome (sensitivity of 0%) (Table 1). In 5 of 10 (50%) of these FN cases, prenatal ultrasound detected the fetal abnormalities and pregnancies were terminated upon confirmation by fetal chromosome analysis. The remaining 5 FN cases were not identified by ultrasound, but were identified at birth and subsequently confirmed by postnatal fetal chromosome analysis. In a 3–6 month follow-up period after birth, no other FN SCAs have been reported and follow-up is continuing.
Potential causes of the ten FNs were further investigated (Table 2). In 9 of 10 pregnancies (exception FN-4 for T18), low fetal DNA fraction was an unlikely cause because FF values were moderate (8–16%). The FN-10 case for CDC was further examined by analysis of the available placental tissue retrieved at TOP. From a single placental biopsy sample, no evidence of a 5p deletion (Fig. 2) was identifiable, suggesting possible placental/fetal mosaicism as a cause of the false negative CDC result.
DISCUSSION
While NIPS has been well accepted by clinicians and patients for detection of common fetal trisomies and SCAs, debate still exists as to the added clinical value of testing for rarer chromosome disease syndromes.25,26 Opponents argue that the relatively low PPVs and the uncertainty of the pathogenicity of many MMS creates difficulty with genetic counseling for high-risk results, increases unnecessary invasive procedures, and transfers undue stress to the couple. On the other hand, proponents argue that the aim of any prenatal diagnosis is to prevent the birth of children with serious chromosome disease syndromes, and even at low to moderate PPVs, NIPS has sufficient sensitivity to identify a significant proportion of fetuses with rare chromosome disease syndromes that escape detection by routine ultrasound scanning. In regard to current NIPS guidelines, the ACMG advocates its use for common aneuploidies, combined with an invasive procedure for all positive findings.24 However, screening for pathogenic or likely pathogenic genome-wide CNVs is currently not recommended by the ACMG. If this level of information is required for management of the pregnancy, then diagnostic testing using CVS or amniocentesis for fetal cell sampling followed by CMA is the ACMG-recommended process. Recently, more relaxed guidelines have been suggested whereby screening for MMS can be performed routinely for younger women where microdeletions are more frequent than aneuploidies.30
Based on its performance in this study of over 94,000 pregnancies, NIPS-Plus displays the hallmarks of a first-tier screening method suitable for the detection of pathogenic aneuploidies, recurrent MMS, and potentially even genome-wide MMS. NIPS-Plus exhibited high sensitivity and specificity for detection of clinically significant microdeletions and duplications while retaining very high sensitivity and specificity for detection of common aneuploidies. The overall PPVs for T21 (95%) and T18 (82%) were high whereas the PPV for T13 (46%) and SCAs (47%) were considered moderate. These PPVs for common aneuploidies are very similar to those reported previously in other clinical follow-up studies.31 The PPVs for rare trisomies were lower at 28% and similar to those reported in a recent study.32 While the causes of all false positive aneuploidies were not investigated further, a possible explanation identified from case studies is confined placental mosaicism (CPM) where observations of the incidence of CPM in typical CVS samples are around 1–2% (ref. 17).
The PPV for DGS in this study was very high (93%). This PPV is significantly higher than what has currently been achieved by other methodologies, with reported PPVs ranging from a relatively low 16–21% in three studies19,21,22 to as high as 71% in one study.20 In one of these patient cohorts19 where the SNP data was retrospectively reanalyzed with deeper sequencing33 the PPV for detection of DGS increased from 15.7% to 44.2%. The overall PPV for detection of other types of more rare MMS was quite variable. For PWS, CDC, and 1p36 del syndrome, the PPVs were 75%, 40%, and 0%, respectively. Similar low PPVs have also been reported for both CDC and 1p36 del syndrome in other studies.22 Intriguingly, in all three false positive calls of CDC reported here, there was a clear and consistent 5pter copy-number loss of the critical deletion interval. Given that a FP case of DGS has previously been associated with low-level CPM,34 we speculate that this may also be a contributing factor in one or more of the 3 FP CDC cases. In regard to other nonsyndromic CNVs detected, the combined PPVs for CNVs >10 Mb (32%) and CNVs <10 Mb (19%) were low but reasonable, indicating possible sufficient sensitivity and specificity of the test for potential screening of genome-wide fetal CNVs.
Overall, the combined frequency of FN for common trisomies and MMS was 0.01%. This included five pregnancies that were detected by ultrasound and five pregnancies detected only at birth. Thus, the simple inclusion of ultrasound would therefore drop the frequency of FNs to 0.005%. In all cases, we were able to show that the basis of the FNs was unlikely to be low fetal DNA fraction (below 3%, which is the lower limit of the test sensitivity) and thus was more likely to have some biological basis not yet considered. One FN case of CDC was further investigated at the placental level, and the finding of no evidence of the 5pter deletion in a placental biopsy specimen identified why the fetal abnormality was essentially undetectable in maternal cfDNA. Based on several isolated FN case NIPS reports,35,36 where low to moderate levels of placental mosaicism were demonstrated, it was hypothesized that mosaicism possibly decreased the effective fetal DNA profile of the abnormality below the limits of detection. Given the lack of availability of placental studies for the other nine FNs in this report, while technical failure of the test cannot be excluded, we speculate that low-level mosaicism may also be a possible explanation in our study. Mosaicism and its impact on the derived fetal profiles will likely remain a biologically based limitation for any indirect methods of fetal assessment such as NIPS. At the time of follow-up, 3–12 months, there were no further FN SCAs reported. To determine true estimates of the FN rate for SCAs, longer follow-up into the early teenage years may be necessary for sex chromosome diseases or mosaic variants to fully manifest and be clinically recognized.
Currently, the state of care for women with a fetal abnormality suspected by either a high-risk maternal serum screen result, soft markers, or ultrasound structural abnormality is invasive prenatal testing.4 Based on the high performance of our expanded screen for detecting a wide range of clinically significant chromosome diseases including MMS, we propose that NIPS-Plus is a candidate for a first-tier screening method for all pregnancies including where a significant risk of occurrence of a chromosome imbalance related to maternal age is absent.3 However, due to unacceptably high false positive rates associated with detection of SCAs and MMS, although lower in this study, the introduction of an expanded first-tier cfDNA screen will result in an increase in unnecessary invasive procedures. Nonetheless, we opt to support the use of NIPS-Plus when the live birth frequency of a SCA or a CNV associated condition reaches or exceeds that of currently screened conditions, even though increasing invasive procedures. As the ACMG states,24 the use of NIPS to include SCA and CNV screening is becoming more commonplace because there are no other screening options available to identify these conditions. Expanded first-tier screening will also offer the possibility of detecting a subset of chromosome disease syndromes that show no visible abnormalities on fetal ultrasound37 including syndromes associated with mental disability, developmental delay, autistic disorders, as well as most SCAs.38 Therefore, with sustained application of NIPS-Plus for first-tier screening of all pregnant women, it may be possible to also have a significant impact on reducing the incidence of newborns with these syndromes.
Given the extreme shortage of trained genetic counselors internationally, there is no doubt that implementation of NIPS-Plus as a first-tier screen into the medical system will be challenging and require reallocation of current resources. Currently, all approaches to prenatal screening and management of patients are associated with an element of diagnostic testing and genetic counseling. For example, traditional FTS currently consumes a huge amount of these resources for what is typically a 95% false positive risk categorization. Thus with increasing use of NIPS-Plus over FTS, NIPS-Plus has the potential to free up more of the available diagnostic testing and counseling resources to appropriately focus on high-risk results. Notwithstanding, if NIPS-Plus was to eventually replace FTS, there would still be an urgent need for more specialized training of current genetic counselors to deal with a whole new range of chromosome disease syndromes detectable by NIPS-Plus in the prenatal period.
In conclusion, this is the first study reported that involved a large prospective group of pregnant women of both mixed general and high-risk classification. As such, the data have potential significance in demonstrating the usefulness of cfDNA profiling not only for common whole chromosome aneuploidies where age is a significant factor but also for CNV changes of clinical significance where age is not considered a significant risk determinant. NIPS-Plus exhibited high performance for detection of trisomies, SCAs, and the most common 22q11.2 microdeletion associated with DiGeorge syndrome, and moderate to low performance for detection of other, genome-wide, segmental imbalances associated with other MMS and some nonsyndromic CNVs. With further clinical experience from the general population of reproductive women, we propose that our NIPS-Plus method, combined with ultrasound as an independent screening system, may eventually have clinical application as the new standard of care for routine screening of pregnancies for fetal pathogenic CNVs associated with chromosome disease syndromes.
References
Vanneste E, Voet T, Le Caignec C, et al. Chromosome instability is common in human cleavage-stage embryos. Nat Med. 2009;15:577–583.
Garcia-Herrero S, Cervero A, Mateu E, et al. Genetic analysis of human preimplantation embryos. Curr Top Dev Biol. 2016;120:421–447.
Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367:2175–2184.
Levy B, Wapner R. Prenatal diagnosis by chromosomal microarray analysis. Fertil Steril. 2018;109:201–212.
Chitty LS, Lo YM. Noninvasive prenatal screening for genetic diseases using massive parallel sequencing of maternal plasma DNA. Cold Spring Harb Perspect Med. 2015;5:a023085.
Song Y, Liu C, Qi H, Zhang Y, Bian X, Liu J. Noninvasive prenatal testing of fetal aneuploidies by massively parallel sequencing in a prospective Chinese population. Prenat Diagn. 2013;33:700–706.
Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799–808.
Mazloom AR, Džakula Ž, Oeth P, et al. Noninvasive prenatal detection of sex chromosomal aneuploidies by sequencing circulating cell-free DNA from maternal plasma. Prenat Diagn. 2013;33:591–597.
Samango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33:643–649.
Wong FC, Lo YM. Prenatal diagnosis innovation: genome sequencing of maternal plasma. Annu Rev Med. 2016;67:419–432.
Benn P, Grati FR. Genome-wide non-invasive prenatal screening for all cytogenetically visible imbalances. Ultrasound Obstet Gynecol. 2018;51:429–433.
Watson CT, Marques-Bonet T, Sharp AJ, Mefford HC. The genetics of microdeletion and microduplication syndromes: an update. Annu Rev Genomics Hum Genet. 2014;15:215–244.
Tézenas Du Montcel S, Mendizabai H, et al. Prevalence of 22q11 microdeletion. J Med Genet. 1996;33:719.
Yu SC, Jiang P, Choy KW, et al. Noninvasive prenatal molecular karyotyping from maternal plasma. PLoS ONE. 2013;8:e60968.
Srinivasan A, Bianchi DW, Huang H, Sehnert AJ, Rava RP. Noninvasive detection of fetal subchromosome abnormalities via deep sequencing of maternal plasma. Am J Hum Genet. 2013;92:167–176.
Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212:332.e331–339.
Grati FR, Malvestiti F, Ferreira JC, et al. Fetoplacental mosaicism: potential implications for false-positive and false-negative noninvasive prenatal screening results. Genet Med. 2014;16:620–624.
Advani HV, Barrett AN, Evans MI, Choolani M. Challenges in non-invasive prenatal screening for sub-chromosomal copy number variations using cell-free DNA. Prenat Diagn. 2017;37:1067–1075.
Dar P, Curnow KJ, Gross SJ, et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am J Obstet Gynecol. 2014;211:527.e521–e517.
Helgeson J, Wardrop J, Boomer T, et al. Clinical outcome of subchromosomal events detected by whole-genome noninvasive prenatal testing. Prenat Diagn. 2015;35:999–1004.
Gross SJ, Stosic M, McDonald-McGinn DM, et al. Clinical experience with single-nucleotide polymorphism-based non-invasive prenatal screening for 22q11.2 deletion syndrome. Ultrasound Obstet Gynecol. 2016;47:177–183.
Petersen AK, Cheung SW, Smith JL, et al. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am J Obstet Gynecol. 2017;217:691.e691–e696.
Schwartz S, Kohan M, Pasion R, Papenhausen PR, Platt LD. Clinical experience of laboratory follow-up with non-invasive prenatal testing using cell-free DNA and positive microdeletion results in 349 cases. Prenat Diagn. 2018;38:210–218.
Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18:1056–1065.
Rose NC, Benn P, Milunsky A. Current controversies in prenatal diagnosis 1: should NIPT routinely include microdeletions/microduplications? Prenat Diagn. 2016;36:10–14.
Evans MI, Wapner RJ, Berkowitz RL. Noninvasive prenatal screening or advanced diagnostic testing: caveat emptor. Am J Obstet Gynecol. 2016;215:298–305.
Kim SK, Hannum G, Geis J, et al. Determination of fetal DNA fraction from the plasma of pregnant women using sequence read counts. Prenat Diagn. 2015;35:810–815.
Liang D, Lv W, Wang H, et al. Non-invasive prenatal testing of fetal whole chromosome aneuploidy by massive parallel sequencing. Prenat Diagn. 2013;33:409–415.
Fromer M, Moran JL, Chambert K, et al. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am J Hum Genet. 2012;91:597–607.
Verma IC, Dua-Puri R, Bijarnia-Mahay S. ACMG 2016 update on noninvasive prenatal testing for fetal aneuploidy: implications for India. J Fetal Med. 2017;4:1–6.
Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell‐free DNA in maternal blood in screening for fetal aneuploidies: updated meta‐analysis. Ultrasound Obstet Gynecol. 2015;45:249–266.
Fiorentino F, Bono S, Pizzuti F, et al. The clinical utility of genome-wide non invasive prenatal screening. Prenat Diagn. 2017;37:593–601.
Martin K, Iyengar S, Kalyan A, et al. Clinical experience with a single-nucleotide polymorphism-based non-invasive prenatal test for five clinically significant microdeletions. Clin Genet. 2018;93:293–300.
Bunnell M, Zhang C, Lee C, Bianchi DW, Wilkins-Haug L. Confined placental mosaicism for 22q11.2 deletion as the etiology for discordant positive NIPT results. Prenat Diagn. 2017;37:416–419.
Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207–1210.
Hochstenbach R, Page-Christiaens GC, van Oppen AC, et al. Unexplained false negative results in noninvasive prenatal testing: two cases involving trisomies 13 and 18. Case Rep Genet. 2015;2015:926545.
Ferreira JC, Grati FR, Bajaj K, et al. Frequency of fetal karyotype abnormalities in women undergoing invasive testing in the absence of ultrasound and other high-risk indications. Prenat Diagn. 2016;36:1146–1155.
Battaglia A, Doccini V, Bernardini L, et al. Confirmation of chromosomal microarray as a first-tier clinical diagnostic test for individuals with developmental delay, intellectual disability, autism spectrum disorders and dysmorphic features. Eur J Paediatr Neurol. 2013;17:589–599.
Acknowledgements
We thank dedicated staff from the Hunan Provincial Maternal and Child Health Care Hospital for assistance with collection of clinical information relevant to this research study. This work was supported by grants from National Key R&D Program of China (numbers 2017YFC1001800/02 and 2016YFC0905100/02), National Natural Science Foundation of China (number 81571450), and Key R&D Program of Hunan Province (number 2017SK2153).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
H.S., Y.Z., F.T., H.Z., M.X., F.Y., and D.S.C. are employees of Berry Genomics Corporation. With the exception of F.T., none of the authors hold any stocks or bonds in the company. The other authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, D., Cram, D.S., Tan, H. et al. Clinical utility of noninvasive prenatal screening for expanded chromosome disease syndromes. Genet Med 21, 1998–2006 (2019). https://doi.org/10.1038/s41436-019-0467-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-019-0467-4
Keywords
This article is cited by
-
Clinical evaluation of noninvasive prenatal testing for sex chromosome aneuploidies in 9,176 Korean pregnant women: a single-center retrospective study
BMC Pregnancy and Childbirth (2024)
-
Clinical outcomes of screen-positive genome-wide cfDNA cases for trisomy 20: results from the global expanded NIPT Consortium
Molecular Cytogenetics (2024)
-
Selection of prenatal screening with nuchal translucency > 95th centile and below 99th centile: a 4-year observational study with real-world data
Archives of Gynecology and Obstetrics (2024)
-
Prospective prenatal cell-free DNA screening for genetic conditions of heterogenous etiologies
Nature Medicine (2024)
-
Evaluation of the clinical utility of extended non-invasive prenatal testing in the detection of chromosomal aneuploidy and microdeletion/microduplication
European Journal of Medical Research (2023)