Abstract
Purpose
We aimed to estimate direct health-care costs and physician utilization for a cohort of children diagnosed with genetic diseases.
Methods
Retrospective cohort study using population-based provincial health administrative data for children with genetic diseases (n = 255) compared with three matched cohorts (asthma n = 1275, diabetes n = 255, general population n = 1275). We estimated direct health-care costs and resource use 5 years after diagnosis in five categories: physician billing, same day surgery, emergency, inpatient hospitalizations, and home care.
Results
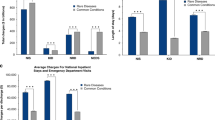
During the postdiagnostic period, annual mean total costs for the genetic disease cohort were significantly higher than all other cohorts. Annual mean total costs for all cohorts were highest in the year after diagnosis with costs for the genetic disease cohort between 4.54 and 19.76 times higher during the 5 years. Inpatient hospitalizations and physician billing accounted for the majority of costs. The genetic disease cohort received more care from specialists, whereas the chronic disease cohorts received more care from general practitioners.
Conclusion
Direct health-care costs for children with genetic diseases are significantly higher than children with/without a chronic disease, particularly in the year after diagnosis. These findings are important when considering resource allocation and funding prioritization for children with genetic diseases.
Similar content being viewed by others
Introduction
Genetic diseases are the direct result of alterations in a person’s genome and include mechanisms such as aneuploidy, small or large deletions/duplications, pathogenic single-nucleotide variants, and other more complex mechanisms such as repeat expansions and imprinting abnormalities. The majority of the 21,000 documented rare diseases in Orphanet are genetic in nature.1 While most of these disorders are individually rare, they are collectively common, affect mostly children, and result in significant morbidity and mortality.2,3
Previous estimates of the costs of caring for individuals with genetic disease have varied depending on disease type, severity, and country of residence.4,5,6 These cost estimates have been restricted to specific rare genetic diseases, limiting their generalizability, and, importantly, some lacked a control group comparator. Robust estimates of the costs of genetic diseases are needed to help health-care policy-makers prioritize and justify policies and funding decisions.4 Once a baseline estimate of direct costs has been established, interventions to inform health-care delivery can then be evaluated both in terms of economic impact and patient experience.4
We used population-based provincial administrative data from Ontario (Canada’s most populous province, whose 13 million inhabitants comprise approximately 40% of the Canadian population)7 to estimate direct health-care costs associated with the care of children diagnosed with genetic diseases. Specifically, we aimed to estimate direct health-care costs and physician utilization for a cohort of children diagnosed with genetic diseases compared with two population-based cohorts of children with other chronic diseases (asthma and diabetes), and a general population cohort.
Materials and methods
We conducted a retrospective cohort study using provincial health administrative data from Ontario, Canada to estimate the direct health-care costs and health services utilization associated with the care of children with selected genetic diseases. These costs were compared with three matched cohorts: children with asthma, children with diabetes, and children from the general population. Ethics approval was from the Children’s Hospital of Eastern Ontario (CHEO) Research Ethics Board (Protocol 11/04E) and University of Calgary Conjoint Health Research Ethics Board (REB15-0722).
Study participants
To identify a representative cohort of patients presenting to clinical genetics, we focused on children with genetic diseases that would be diagnosed in a typical genetics clinic. Provincial privacy legislation stipulates that there must be six or more patients for each disease for aggregate reporting of administrative data. We identified the 17 genetic diseases seen over our data capture time frame that met this criteria; these ranged in prevalence from 1:1000 (Down syndrome) to 1:40,000 (Dravet syndrome) and included both static and progressive diseases (Table 1). In addition, we added a disease category to collectively represent ultrarare diseases with an estimated prevalence <1:100,000. We queried the internal database in the Department of Genetics at CHEO and included all patients who met our inclusion criteria: younger than 16 years of age at first visit, diagnosed with one of the specified genetic diseases with a confirmed molecular diagnosis, and seen by a physician between 1 April 2002 and 31 March 2014. CHEO is the only pediatric health-care facility in Eastern Ontario and has a catchment of 1.2 million people; therefore patients evaluated in the Department of Genetics represent a population-based sample of patients from the region. We conducted a retrospective chart review to confirm their clinical/molecular diagnoses and collect demographic information. Patients were excluded if they were not a resident of Ontario, were not covered by the Ontario Health Insurance Plan (OHIP) for medical services (i.e., recent immigrants within 3 months of arrival, self-paid, status First Nations/Inuit with health coverage by the federal government, or military families with Canadian Forces health coverage), survived <24 hours, and/or had insufficient clinical information in their chart to confirm eligibility.
We linked the data for eligible patients for the genetic disease cohort to administrative databases using their OHIP number. We defined the index event date for patients with a genetic disease as the date of diagnosis (determined from chart review). If the date of diagnosis was prior to the patient’s date of birth (i.e., the patient was molecularly diagnosed prenatally), then the birth date served as the index event date.
Comparison cohorts
Our comparison cohorts included children with other chronic diseases (asthma or diabetes) and those from the general population. We identified children <18 years with asthma from the Ontario Asthma Dataset using a validated algorithm with 89% sensitivity and 72% specificity.8 We identified children <19 years with type 1 diabetes from the Ontario Diabetes Database using a validated algorithm with 83% sensitivity and 99% specificity.9 The general population cohort was identified by randomly selecting patients from Ontario health administrative data and then matching criteria were applied to make the cohorts more comparable. To compare the cohorts over time, the index event date was the date of diagnosis for the chronic disease cohorts as defined by the validated algorithms.8,9,10 For the general population cohort, we defined their index event date as the same age as their matched genetic patient’s age at diagnosis. We matched the comparison cohorts to the genetic disease cohort based on sex, date of birth (+/− 1 year for asthma and general population and +/− 2 years for diabetes), income quintile (high: quintiles 3–5; low: quintiles 1–2),11,12 rural versus urban household at birth,13 and index event date (+/− 1 year asthma and +/− 1.5 years for diabetes). We were able to match five patients for each patient with genetic disease in the asthma and general population cohorts, but only one patient with diabetes could be matched with each patient with genetic disease. While no patient was in more than one cohort, patients could have more than one health condition; for example, some patients in the diabetes cohort also had asthma.
Time frames
We analyzed the data using a look-forward observation window of a maximum of 5 years relative to their diagnosis date. The look-forward window was terminated before 5 years if the study end date was reached or if the patient became ineligible to receive OHIP. In the latter situation, the patient was followed until OHIP coverage was terminated for the first time, starting from the date of diagnosis. Follow-up was also terminated if the matches became ineligible or died.
Administrative data sources
The province of Ontario has a universal health-care system in which all legal residents of the province with OHIP coverage have access to government-funded nonelective outpatient, emergency, and hospital care.14 More than 99% of non–First Nations Ontario residents have OHIP coverage and are included in the administrative data. First Nations peoples living on reservations are not covered by OHIP and are covered by a federal health plan. Thirty-seven percent of Ontario’s 125,000 First Nations peoples live on reservations and would not be included in the administrative data (less than 0.5% of the Ontario population).15
All health-care encounters and charges to the Ontario health system are contained within health administrative data and cost analyses, including physician visits (including pediatrician and other specialist physicians), same day surgery, emergency department (ED) utilization, inpatient hospitalizations, laboratory procedures, and home care.16,17 These data are housed by the Institute for Clinical Evaluative Sciences (ICES) through an agreement with the Ontario Ministry of Health and Long-Term Care (MOHLTC).18 We used the patients’ OHIP number, derived from chart review of patients with genetic disease as described above, to deterministically link to encrypted patient identification numbers at ICES. The health administrative databases used are detailed in Supplementary Table 1.
Direct health-care costs and resource use
We estimated annual mean total direct health-care costs per patient using a validated costing methodology developed for Ontario health administrative data that captures costs paid by the Ontario MOHLTC.16 With the exception of OHIP physician billings, visit-level costs for other health sector categories were calculated by multiplying the visit resource intensity weight (RIW) by a province-wide measure of cost per weighted cases (CPWC). The RIW is a measure of resource usage for a specific visit relative to the resource usage of an average visit, and CPWC is a measure of the dollars required to care for the average patient. These RIWs and CPWCs are year and sector-specific. Costs were prorated to the appropriate period, whenever an admission overlapped with multiple periods. All costs were adjusted for inflation to 2012 Canadian dollar values using the health-care specific yearly Consumer Price Index reported by Statistics Canada.19 Costing variables across all health sector categories were only available starting in fiscal year 2003. Therefore, only patients with an index visit post-2003 were included. All costs are reported in Canadian dollars (CAD).
We estimated mean direct health-care costs stratified by category 1 year after diagnosis date. There were five cost categories: physician billing, same day surgery, emergency department, inpatient hospitalizations, and home care. We compared these estimates for each of the matched cohorts. Finally, we captured patterns of physician utilization (family physician or specialist physician, including pediatricians) after diagnosis and compared these patterns for each of the matched cohorts.
Statistical analyses
Descriptive statistics were reported for demographic variables using means (with SD of the sample), medians (with interquartile range [IQR]), or proportions as appropriate. Means were used to report event rates in the genetic disease cohort after diagnosis and to compare the cohorts at specific time points (e.g., 1 year after diagnosis). All confidence intervals were at the 95% confidence level (95% CI). Although most frequency and cost distributions in the analysis were skewed (a small proportion of patients had high costs), the large sample size allowed for inference on the mean.
To compare mean total cost between cohorts, cost ratios were calculated for each year after diagnosis by dividing the mean total cost per patient in each cohort: for example, the mean total cost of the genetic disease cohort matched to asthma cohort divided by the mean total cost of the asthma cohort to estimate the cost ratio of the mean total cost for the genetic versus asthma cohort. Similarly, the cost ratio was calculated for each of the five health cost categories 1 year after diagnosis date. We computed 95% CIs for these cost ratios using a delta method approximation.20
All analyses were performed using SAS Enterprise Guide version 7.3 (SAS Corp., Cary, NC).
Results
There were 255 patients in the genetic disease cohort, 1275 in the asthma cohort, 255 in the diabetes cohort, and 1275 in the general population cohort. Table 2 summarizes the genetic disease cohort demographics and characteristics. The mean age at diagnosis was 3.2–3.3 years for all cohorts, with most being younger than 4 years; only 4% were over 15 years of age. The diagnosis was made in the first year of life for 120 (of 255; 47%) patients in the genetic disease cohort; approximately 30% (n = ~80) had a progressive disease, the remainder (n = ~170) had a static disease (numbers are approximate because cell sizes <6 were suppressed per privacy legislation).
Direct health-care costs and comparisons by cohort
During the entire postdiagnostic period, annual mean total costs of health care for a child with genetic disease were significantly higher than the annual mean total costs of care for a child with other chronic diseases (asthma or diabetes) and from the general population (Table 3, Supplementary Table 2). There were two exceptions to this: although the cost for the genetic disease cohort was higher than the matched diabetes cohort, the cost ratios in years 2 and 3 after diagnosis did not differ statistically. The annual mean total costs for the genetic disease cohort matched to the general population were between 4.54 and 19.76 times higher (Table 3) for 5 years following diagnosis, with the highest cost ratio in the year after diagnosis. Even when compared with the other chronic disease cohorts, the annual mean total costs for the genetic disease cohort were higher: 3.50 to 8.27 times higher than asthma and 1.77 to 7.33 times higher than diabetes. The highest annual mean total cost for all cohorts occurred in the year after diagnosis: CAD$23,333 for the genetic disease cohort compared with CAD$3316, CAD$4890, and CAD$1181 for the asthma, diabetes, and general population cohorts, respectively (Table 3, Supplementary Table 2). Across the genetic disease and chronic disease cohorts, the annual mean total costs were lower in subsequent years compared with the year after diagnosis (Table 3, Supplementary Table 2).
Comparison of direct health-care costs by category
Across all five cost categories (physician billing, same day surgery, emergency department, inpatient hospitalizations, and home care), mean costs for the genetic disease cohort were significantly higher than for the matched chronic disease cohorts and the general population cohort (Table 4, Supplementary Table 3). There were two exceptions to this: although the cost for emergency department visits for the genetic disease cohort were higher than for the matched asthma and diabetes cohorts, the cost ratios did not differ statistically. Inpatient hospitalizations and physician billing accounted for the majority of costs for the genetic disease cohorts as well as the comparison cohorts. For example, for the genetic disease cohort matched to the asthma cohort, the mean inpatient hospitalization cost was CAD$18,313, followed by the mean physician billing cost (CAD$4420) and the mean home care cost (CAD$3746). The remaining mean costs for same day surgery and the emergency department were less than CAD$400 each.
In the year after diagnosis, the cost ratios associated with the care of children with genetic disease in the various cost categories ranged from 1.16 (emergency department) to 13.56 (home care) when matched to the asthma cohort, from 1.24 (emergency department) to 6.30 (same day surgery) when matched to the diabetes cohort, and from 3.97 (emergency department) to 156.41 (home care) when matched to the general population cohort (Table 4, Supplementary Table 3).
Patterns of physician utilization
Children in the genetic disease cohort received more care from specialists (including pediatricians), whereas children in the chronic disease cohorts received more care from family physicians. In the first year after diagnosis, children in the genetic disease cohort saw a specialist for 25.79 (95% CI: 21.60–29.80) visits a year on average. This was higher than for the asthma, diabetes, and general population cohorts who, in the first year after diagnosis, sought specialist care an average of 6.06 (95% CI: 5.58–6.54), 7.22 (95% CI: 5.89–8.55), and 2.47 (95% CI: 2.15–2.79) times a year, respectively. After 5 years of follow-up, all cohorts saw a decrease in specialist visits, but the genetic disease cohort still received more specialist care on average, with 9.18 (95% CI: 7.14–11.22) visits per year compared with 4.86 (95% CI: 3.11–6.61) for diabetes patients, who experience the second highest visit rate. Noteworthy is that the genetic disease cohort received more specialist care in the first year than in the second year, where the mean number of visits was reduced by half to 13.16 (95% CI: 10.89–15.43) visits per year.
In contrast, the comparison cohorts received more care from family physicians. In the first year after diagnosis, the asthma, diabetes, and general population cohorts saw a family physician an average of 5.95 (95% CI: 5.63–6.27), 5.53 (95% CI: 4.86–6.20), and 4.37 (95% CI: 4.11–4.63) times, respectively, whereas the genetic disease cohort saw a family physician only 3.63 (95% CI: 2.86–4.40) times per year on average. In the 5-year follow-up, all patients experienced a decrease in their mean number of family physician visits.
Discussion
This study provides a robust assessment of the direct costs and resource use associated with the health care of children diagnosed with genetic diseases. We used population-based health administrative data to estimate costs and resource use associated with the care of children affected by a subset of representative genetic diseases. The costs associated with the care of these children were compared with those for two population-based cohorts of children with other chronic diseases (asthma and diabetes) and a general population cohort, which were matched on sex, date of birth, income quintile, rural versus urban household at birth, and index event date. Our data captured direct costs and resource use up to 5 years after diagnosis, including physician billings, same day surgeries, emergency department visits, inpatient hospitalizations, home care, and physician utilization.
Several prominent themes emerged from our analysis: overall, the annual mean total direct health-care costs for children with genetic diseases were considerable and consistently greater than those for the three comparator cohorts. For 5 years after diagnosis, annual mean total costs associated with care for children with genetic disease were 4.54 to 19.76 times greater than the general population, and 1.77 to 8.27 times greater than those with other chronic disease (asthma or diabetes). Annual mean total costs for all cohorts were highest in the first year after diagnosis (CAD$23,333) before decreasing in the subsequent 4 years.
The main source of costs the year after diagnosis for all cohorts was inpatient hospitalizations and physician billings. This was especially marked in our genetic disease cohort, which could be explained, in part, by the fact that 47% (n = 120) of the children included in the cohort were diagnosed in the first year of life. This subgroup of patients contains highly recognizable genetic diseases (e.g., Down syndrome and CHARGE syndrome), which are associated with congenital malformations that require intervention and may inflate the costs in the short term postdiagnosis. These findings are consistent with other studies; for example, more than half of inpatient admissions for Down syndrome (who comprised 28% of our genetic cohort) occur during the first year of life,21 high levels of inpatient care, diagnostic procedures and health-care provider visits have been reported in the first year of life of infants with CHARGE syndrome,22 and patients with cystic fibrosis use a considerable amount of inpatient care.4
We observed that children with genetic disease were more likely to visit specialists and less likely to visit family physicians than the three comparator cohorts over the 5 years after diagnosis. Specialist visits for children with genetic disease were highest in the year after diagnosis (~26 visits per year, on average), decreasing sharply by the second year then remaining relatively stable for the remaining 5 years. This is consistent with a study of 179 children with tuberous sclerosis reporting frequent physician visits (24 visits per year, on average) that were often specialists such as pediatric neurologists, pediatricians, ophthalmologists, neurologists/neurosurgeons, dermatologists, or cardiologists.23
To our knowledge, our study is the first to examine direct health-care costs in a population-based sample of children with genetic diseases matched to children with chronic disease or those from the general population. Previous research exploring the costs of specific rare genetic diseases found that costs vary depending on disease type, severity, and country of residence of the patient. For example, a systematic review examining the direct costs (including inpatient care, medical equipment, and medication) for ten rare diseases, the majority of which were genetic diseases, reported annual per-patient costs ranging from €1042 to €745,376, with the highest costs associated with medications.4 However, it is difficult to make comparisons as this review included a variety of costs that are challenging to align with our data, and with the exception of cystic fibrosis, most studies were limited and outdated. A US study examining the costs of genetic disease associated with inpatient care reported the average per-patient cost per admission (from 3.5 to 7.6 days) ranged from US$8904 to US$17,290.6 This is greater than our annual estimated costs for similar patients because that study was focused only on inpatients, whereas our study focused on a population-based sample. Finally, a Canadian study of direct health-care costs in Ontario (using ICES data) found that medical complexity in children resulted in mean and median direct costs of CAD$53,188 and CAD$17,372 per patient, respectively, over a 2-year period following hospitalization.5 Investigators used an algorithm to identify children with complex chronic health conditions that are typically associated with significant functional status limitations (including genetic disease). Direct costs were similar to those observed 1 year after diagnosis in our study (physician billing, inpatient hospitalizations, home care, emergency department visits, and same day surgery). Importantly, many of these published studies lack comparators, which is a key strength of our study design. Our findings confirm that annual mean total costs of health care for a child with genetic disease were significantly higher compared with a child with other chronic diseases and from the general population, particularly in the year after diagnosis.
While our study is a large population-based analysis examining direct costs and resource use in the genetic disease population, our results must be considered in the context of certain limitations. First, our genetic disease cohort was limited to a sample of patients from one center. However, this center is the only pediatric health-care facility in eastern Ontario and therefore included patients who represent a population-based sample of individuals from the region. Because pediatric health-care services are similarly centralized in other provinces, we would expect that similar patterns regarding the relationship of costs for children diagnosed with genetic diseases compared with children with other chronic diseases would be similar, even though the actual costs would likely differ. Additionally, our sample of genetic diseases is a subset of all genetic diseases and did not include all diseases diagnosed at the center. Our genetic disease cohort only included diseases with a clinical/molecular diagnosis and did not include children without a diagnosis for their condition. Future research should explore the variation in costs and health-care resource use between children with readily diagnosed genetic diseases versus children with ultrarare genetic diseases versus children with undiagnosed genetic disease. There is also a risk of differential misclassification bias, because the children with genetic diseases were identified from a clinical database, while the children in our comparator cohorts were ascertained through health administrative data, albeit using validated algorithms. In addition, the number of children available for follow-up for 5 full years after diagnosis was small, complicating the comparison of data from these periods with data from periods closer to time of diagnosis.
Secondly, the costs included in our analyses represent direct health-care costs based on the utilization of health services, and do not include medication, indirect (e.g., absenteeism) and out-of-pocket costs (costs borne directly by patients that are not covered by government health care or private insurance, such as the costs of nonprescription drugs and devices, copay or deductibles, and complementary/alternative therapies), or allied health services (e.g., physiotherapy, occupational therapy, speech therapy). At the time of the study, prescription medication records were only available in Ontario health administrative data for children who received social assistance; because they were not population based, they were excluded from cost calculations. Out-of-pocket costs for health services and medications are an important consideration from the perspective of patients. A recent Canadian study of children with intellectual developmental disorder (many of whom would have a genetic disease) estimated annual out-of-pocket costs to parents, and non–health system costs, to be CAD$44,570 and CAD$27,428, respectively,24,25 highlighting the significant economic burden on families that would not be captured by our study.
Our results demonstrate that children diagnosed with genetic diseases have higher direct health-care costs with the greatest annual mean costs occurring the year after diagnosis. Children diagnosed with genetic diseases visit specialists more often than children diagnosed with asthma or diabetes or children in the general population, but visit family physicians less often. Future research should explore the variation in costs and health-care resource use between children with readily diagnosed genetic diseases versus children with ultrarare genetic diseases versus children with undiagnosed genetic disease, as well as differences between static and progressive diseases. Ultimately, we believe these estimates of the cost of genetic diseases compared with matched cohorts of children with chronic disease and children from the general population will have important implications for resource allocation and funding prioritization in this population.
References
Orphanet. Rare diseases. 2018. http://www.orpha.net/cgi-bin/cgi-bin/Disease_Search_List.php?lng=EN&TAG=0. Accessed 25 January 2018.
Orphanet. Orphanet report series—rare diseases collection. Prevalence and incidence of rare diseases: bibliographic data. June 2017. http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_diseases.pdf.
Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14:681–691.
Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy. 2015;119:964–979.
Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130:e1463–e1470.
McCandless SE, Brunger JW, Cassidy SB. The burden of genetic disease on inpatient care in a children’s hospital. Am J Hum Genet. 2004;74:121–127.
Statistics Canada. CANSIM, table 051-0001. 2016. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm. Accessed 23 August 2017.
Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16:183–188.
Guttmann A, Nakhla M, Henderson M, et al. Validation of a health administrative data algorithm for assessing the epidemiology of diabetes in Canadian children. Pediatr Diabetes. 2010;11:122–128.
Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516.
Statistics Canada. Postal code conversion file plus (PCCF+) (82F0086X). 2017. http://www5.statcan.gc.ca/olc-cel/olc.action?objId=82F0086X&objType=2&lang=en&limit=0. Accessed 24 May 2017.
Wilkins R, Berthelot J, Ng E. Trends in mortality by neighbourhood income in urban Canada from 1971 to 1996. Health Rep. 2002;13 Suppl:1–27.
Kralj B Measuring rurality—RIO2008_BASIC: methodology and results. Toronto, Ontario, Canada, Ontario Medical Association Economics Department. 2009.
Ontario Ministry of Health and Long-Term Care. Ontario Health Insurance Plan—questions and answers. 2015. http://www.health.gov.on.ca/en/public/programs/ohip/ohipfaq_mn.aspx. Accessed 14 June 2018.
Statistics Canada. Distribution of First Nations people, First Nations people with and without registered Indian status, and First Nations people with registered Indian status living on or off reserve, Canada, provinces and territories. 2011. https://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-011-x/2011001/tbl/tbl03-eng.cfm. Accessed 14 June 2018.
Wodchis W, Bushmeneva K, Nikitovic M, McKillop I Guidelines on person-level costing using administrative databases in Ontario. Toronto, ON, Health System Performance Research Network. 2013.
Government of Ontario. What OHIP covers. 2018. https://www.ontario.ca/page/what-ohip-covers. Accessed 14 June 2018.
Institute for Clinical Evaluative Sciences (ICES). Data dictionary. 2017. https://datadictionary.ices.on.ca/Applications/DataDictionary/Default.aspx. Accessed 28 June 2017.
Statistics Canada. Consumer Price Index, health and personal care, by province (Canada). 2017. Statistics Canada, CANSIM, table 326-0021 and catalogue nos. 0062-0001-X and 0062-0010-X. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ161a-eng.htm. Accessed 24 May 2017.
Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32.
Dawson AL, Cassell CH, Oster ME, et al. Hospitalizations and associated costs in a population-based study of children with Down syndrome born in Florida. Birth Defects Res A Clin Mol Teratol. 2014;100:826–836.
Anderzén-Carlsson A. CHARGE syndrome—a five case study of the syndrome characteristics and health care consumption during the first year in life. J Pediatr Nurs. 2015;30:6–16.
Rentz AM, Skalicky AM, Liu Z, et al. Tuberous sclerosis complex: a survey of health care resource use and health burden. Pediatr Neurol. 2015;52:435–441.
Generaux D, van Karnebeek CDM, Birch PH. Costs of caring for children with an intellectual developmental disorder. Disabil Health J. 2015;8:646–651.
van der Burgt I. Noonan syndrome. Orphanet J Rare Dis. 2007;2:4–4.
Acknowledgements
Care4Rare Canada consortium is funded by Genome Canada, Canadian Institutes of Health Research, Ontario Genomics Institute, Ontario Research Fund, Genome Quebec, and Children’s Hospital of Eastern Ontario Foundation. The study sponsors did not have a role in study design; collection, analysis, interpretation of data; writing the manuscript; or the decision to submit the manuscript for publication. E.I.B. is supported by a New Investigator Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology, Crohn’s and Colitis Canada, and the Career Enhancement Program of the Canadian Child Health Clinician Scientist Program. D.M. is Canada Research Chair (Health Systems and Services Research) and Arthur J.E. Child Chair in Rheumatology. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario MOHLTC. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at http://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Marshall, D.A., Benchimol, E.I., MacKenzie, A. et al. Direct health-care costs for children diagnosed with genetic diseases are significantly higher than for children with other chronic diseases. Genet Med 21, 1049–1057 (2019). https://doi.org/10.1038/s41436-018-0289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-018-0289-9
Keywords
This article is cited by
-
The expanding diagnostic toolbox for rare genetic diseases
Nature Reviews Genetics (2024)
-
Rapid genomic sequencing for genetic disease diagnosis and therapy in intensive care units: a review
npj Genomic Medicine (2024)
-
Are We Capturing the Socioeconomic Burden of Rare Genetic Disease? A Scoping Review of Economic Evaluations and Cost-of-Illness Studies
PharmacoEconomics (2023)
-
Developing a Framework of Cost Elements of Socioeconomic Burden of Rare Disease: A Scoping Review
PharmacoEconomics (2023)
-
Evaluation of Real-World Healthcare Resource Utilization and Associated Costs in Children with Juvenile Idiopathic Arthritis: A Canadian Retrospective Cohort Study
Rheumatology and Therapy (2021)