Abstract
Objective
To study the impact of definitions of various treatment extension criteria on the proportion of patients who could be extended at their first visit after the loading phase of 2 mg aflibercept therapy for neovascular age related macular degeneration (nAMD).
Methods
Patients with nAMD initiated on the loading phase of three intravitreal doses of 2 mg aflibercept in routine clinical practice were recruited from December 2019 to August 2021. The response to the loading phase was assessed at approximately 8 weeks post-loading (up to 140 days from first injection) based on different definitions of response. The proportion of patients that qualify for interval extension based on different clinical trial criteria was also evaluated.
Results
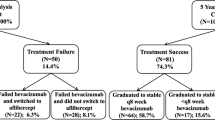
A total of 722 patients with visual acuity (VA) and optical coherence tomography (OCT) scans done at all 4 visits were included. Of these 32.4% of eyes responded with complete macular fluid resolution after the first injection with no recurrence through the loading phase (super-responders) while 26.9% had persistent macular fluid in all 4 visits (true non-responders). The rest were considered suboptimal responders. Change in VA showed marked variations within each of these categories of fluid resolution. For extension of next treatment interval, if presence of any macular fluid at the post-loading visit is the only criteria considered, about 50% could be extended to 8 weeks. If both VA worsening by ≥5 letters and a > 25 μm increase in central sub-field thickness (CST) are considered, 90% will be eligible for interval extension.
Conclusion
Clinical trial designs and pre-defined treatment extension/shortening criteria determine the proportion of patients requiring treatment in the post-loading visit. The short and long-term impact of interval extension immediate post-loading on visual outcome in clinical practice is unknown.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–4.
Fong AH, Lai TY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clin Inter Aging. 2013;8:467–83.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58.
Richard G, Mones J, Wolf S, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology. 2015;122:2497–503.
Stewart MW. Individualized treatment of neovascular age-related macular degeneration: what are patients gaining? or losing? J Clin Med. 2015;21:1079–101.
DeCroos FC, Reed D, Adam MK, Salz D, Gupta OP, Ho AC, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142–50.
Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study: a randomized clinical trial. Retina. 2021;1:1911–20.
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR : a randomized controlled trial. Adv Ther. 2020;37:1173–87.
Lanzetta P, Korobelnik JF, Heier JS, Leal S, Holz FG, Clark WL, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024;403:1141–52.
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;19:729–40.
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84.
Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye. 2015;29:1397–8.
Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–36.
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411.
Kodjikian L, Souied EH, Mimoun G, Mauget-Faÿsse M, Behar-Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology. 2013;120:2300–9.
Busbee BG, Ho AC, Brown DM, Heier JS, Suñer IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56.
Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–52.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmolog. 2015;122:2514–22.
K. Kertes PJ, Galic IJ, Greve M, Williams RG, Rampakakis E, Scarino A, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology. 2019;126:841–8.
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Ng P. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137:372–9.
Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmology. 2019;126:1141–54.
Kikushima W, Sakurada Y, Yoneyama S, Sugiyama A, Tanabe N, Kume A, et al. Incidence and risk factors of retreatment after three-monthly aflibercept therapy for exudative age-related macular degeneration. Sci Rep. 2017;7:44020.
Ohji M, Okada AA, Sasaki K, Moon SC, Machewitz T, Takahashi K, et al. Relationship between retinal fluid and visual acuity in patients with exudative age-related macular degeneration treated with intravitreal aflibercept using a treat-and-extend regimen: subgroup and post-hoc analyses from the ALTAIR study. Graefes Arch Clin Exp Ophthalmol. 2021;259:3637–47.
Jaffe GJ, Kaiser PK, Thompson D, Gibson A, Saroj N, Vitti R, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology. 2016;123:1856–64.
Chakravarthy U, Havilio M, Syntosi A, Pillai N, Wilkes E, Benyamini G, et al. Impact of macular fluid volume fluctuations on visual acuity during anti-VEGF therapy in eyes with nAMD. Eye. 2021;35:2983–90.
Lai TT, Hsieh YT, Yang CM, Ho TC, Yang CH. Biomarkers of optical coherence tomography in evaluating the treatment outcomes of neovascular age-related macular degeneration: a real-world study. Sci Rep. 2019;J9:529.
Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:1860–70.
Waldstein SM, Simader C, Staurenghi G, Chong NV, Mitchell P, Jaffe GJ, et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology. 2016;123:1521–9.
Funding
The investigator-initiated study was funded by Boehringer Ingelheim International GmbH (Grant number: SIVS1045). This study was also supported by the NIHR Biomedical Research Centre and Clinical Research Facility at the Moorfields Eye Hospital National Health Service Foundation Trust and the University College London Institute of Ophthalmology. Boehringer Ingelheim reviewed and approved submission of the manuscript. Benjamin Burton has been on advisory boards and received international conference attendance sponsored by Novartis, Roche and Bayer. Geeta Menon has conducted consultancy-advisory boards for Novartis, Bayer and Allergan, received educational travel grants from Novartis, Bayer, Allergan. Ian Pearce has received lecture fees from Allergan, Bayer, Roche, Heidelberg and Novartis, consultancy fees from Allergan, Alimera, Bayer and Novartis and travel fees from Allergan, Bayer and Novartis. Faruque Ghanchi has received honorarium for consultancy-advisory boards from Alimera, Allergan, Bayer, Novartis, Oxford Bioelectronics, Roche, educational travel grants from Allergan, Bayer, Novartis. Faruque Ghanchi is a section editor of EYE. Martin McKibbin has received consultancy, lecture or advisory board honoraria from Bayer, Novartis and Riche and educational travel grants from Bayer, Novartis and Roche. Praveen J. Patel has received lecture and advisory board honoraria from Bayer, Novartis and Roche and educational travel grants from Bayer and Roche. Richard Gale has conducted consultancy-advisory boards for Novartis, Bayer, Biogen, Boehringer Ingelheim and Allergan, Alimera, Santen, received educational travel grants from Novartis, Bayer, Allergan, Heidelberg Engineering. James Talks is a consultant for Bayer and Roche, received grant support from Bayer, Novartis and Heidelberg Engineering, and is involved in research for Allergan, Alimera, Roche, Bayer, Novartis and Boehringer-Ingelheim. Ajay Kotagiri received travel support from Novartis, Bayer, and Allergan, and speaker fees from Allergan and Bayer. Shruti Chandra received travel support from Boehringer Ingelheim and is an editorial board member of EYE. Sivaprasad reported receiving financial support from AbbVie, Amgen, Apellis, Bayer, Biogen, Boehringer Ingelheim, Novartis, Eyebiotech, Eyepoint Pharmaceuticals, Janssen Pharmaceuticals, Nova Nordisk, Optos, Ocular Therapeutix, Kriya Therapeutics, OcuTerra, Roche, Stealth Biotherapeutics and Sanofi. Sobha Sivaprasad is the Editor-in-Chief of EYE.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.T. and S.S.; Data curation: Sh.C., S.T., E.K. and S.C.; Formal analysis: S. T, S.G. and S.S.; Funding acquisition: S.S.; Methodology: S.T, S.G. and S.S; Project administration: S.S.; Resources: S.T., G.M., B.J.B., I.P., M.M., Sh.C., S.C., A.K., J.T., A.G., F.G., R.G., L.K. and S.S.; Supervision: An.G., S.S.; Visualization: S.G. and S.T.; Writing – original draft: S.T, S.G. and S.S.; Writing - review & editing: S.S. Review and approval of final manuscript: S.T., S.G., An.G., S.S, P.J.P., G.M., B.J.B., I.P., M.M., S.T.,S.C., A.K., J.T., F.G., R.G. and E.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thottarath, S., Gurudas, S., Chandak, S. et al. Impact of treat and extend criteria on proportions that can be extended after loading phase of 2 mg aflibercept therapy for neovascular age related macular degeneration: PRECISE Report 5. Eye (2024). https://doi.org/10.1038/s41433-024-03110-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-024-03110-4