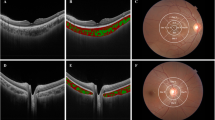
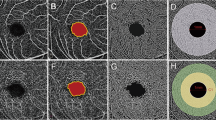
Abstract
Aims
To compare the vascular density (VD) of the macular superficial capillary plexus (SCP), deep capillary plexus (DCP), and choriocapillaris (CC), and the foveal avascular zone (FAZ) among high hyperopic, high myopic, and emmetropic children using optical coherence tomography angiography (OCTA).
Methods
This was a cross sectional comparative study of otherwise healthy children with different refractive errors. Patients were recruited from Cairo University Children’s Hospital. OCTA imaging was performed using the RTVue XR Avanti device with AngioVue software. Both the 3 × 3 and 6 × 6 mm macular scans were utilized. Automated measurements were obtained from the built-in machine software. Axial length (AL) measurements were done using Lenstar LS 900 optical biometer.
Results
Ninety eyes from 51 healthy children were included. Among high myopes, there was significant thinning of the parafovea (p < 0.001). SCP-VD was also lower in high myopes in all areas except the fovea (all p < 0.001). The DCP-VD was significantly lower in high myopes in the parafovea and perifovea. High hyperopes had lower subfoveal CC-VD. Despite high myopes showing a significantly lower OCTA signal strength, linear regression analysis revealed that AL was an independent and significant predictor for the FAZ-area, as well as parafoveal and perifoveal SCP and DCP-VD.
Conclusion
High myopia results in a reduction of VD in both the SCP and DCP, which can be non-invasively detected and monitored using OCTA. While lower VD may, in part, be attributed to lower OCTA image quality, our findings demonstrate that AL independently and significantly predicts macular vascular parameters on OCTA in children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Data is available from the corresponding author upon reasonable request.
References
Elnahry AG, Khafagy MM, Esmat SM, Mortada HA. Prevalence and associations of posterior segment manifestations in a cohort of Egyptian patients with pathological myopia. Curr Eye Res. 2019;44:955–62.
Venkatesh R, Sinha S, Gangadharaiah D, Gadde SGK, Mohan A, Shetty R, et al. Retinal structural-vascular-functional relationship using optical coherence tomography and optical coherence tomography - angiography in myopia. Eye Vis (Lond). 2019;6:8.
Sasaki K, Sasaki K, Hirota M, Hayashi T, Mizota A. Comparisons of size of foveal avascular zone area among children with posterior microphthalmos, high hyperopia, and normal eyes. Int Ophthalmol. 2022; https://doi.org/10.1007/s10792-022-02250-4. PMID: 35366136.
Flitcroft DI. Emmetropisation and the aetiology of refractive errors. Eye. 2014;28:169.
Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821.
Man RE, Lamoureux EL, Taouk Y, Xie J, Sasongko MB, Best WJ, et al. Axial length, retinal function, and oxygen consumption: a potential mechanism for a lower risk of diabetic retinopathy in longer eyes. Investig Ophthalmol Vis Sci. 2013;54:7691–8.
Ong SY, Ikram MK, Haaland BA, Cheng CY, Saw SM, Wong TY, et al. Myopia and cognitive dysfunction: the singapore malay eye study. Invest Ophthalmol Vis Sci. 2013;54:799–803.
Chew EY. The Landmark Beaver Dam Eye study ushered in modern epidemiologic research in age-related macular degeneration. Ophthalmol. 2020;127:S120–21.
Li H, Mitchell P, Rochtchina E, Burlutsky G, Wong TY, Wang JJ. Retinal vessel caliber and myopic retinopathy: the blue mountains eye study. Ophthalmic Epidemiol. 2011;18:275–80.
Al-Sheikh M, Falavarjani KG, Pfau M, Uji A, Le PP, Sadda SR. Quantitative features of the choriocapillaris in healthy individuals using swept-source optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retin. 2017;48:623–31.
Fan H, Chen HY, Ma HJ, Chang Z, Yin HQ, Ng DS, et al. Reduced macular vascular density in myopic eyes. Chin. Med. J. 2017;130:445–51.
Suwan Y, Fard MA, Geyman LS, Tantraworasin A, Chui TY, Rosen RB, et al. Association of myopia with peripapillary perfused capillary density in patients with glaucoma: an optical coherence tomography angiography study. JAMA Ophthalmol. 2018;136:507–13.
Elnahry AG, Noureldine AM, Abdel-Kader AA, Sorour OA, Ramsey DJ. Optical coherence tomography angiography biomarkers predict anatomical response to bevacizumab in diabetic macular Edema. Diabetes Metab Syndr Obes. 2022;15:395–405.
Demircan A, Yesilkaya C, Altan C, Alkin Z, Yasa D, Aygit ED, et al. Foveal avascular zone area measurements with optical coherence tomography angiography in patients with nanophthalmos. Eye (Lond). 2018; https://doi.org/10.1038/s41433-018-0236-7.
Funakoshi S, Yoshikawa T, Harada Y, Chikama T, Kiuchi Y. Absence of the foveal avascular zone in a nanophthalmic child revealed by optical coherence tomography angiography. Am J Ophthalmol Cas Rep. 2019;13:34–37.
Sargues LR, Adsuara CM, Navarro VC, Palop CN, Hernández JM, Sanchis MIS, et al. Quantitative analysis of retinal and choroidal vasculature in patients with chorioretinal folds secondary to hyperopia. Euro J Ophthalmol. 2021; 32. https://doi.org/10.1177/11206721211035618.
Gołębiewska J, Biała-Gosek K, Czeszyk A, Hautz W. Optical coherence tomography angiography of superficial retinal vessel density and foveal avascular zone in myopic children. PLoS One. 2019;14:e0219785. https://doi.org/10.1371/journal.pone.0219785.
Zhu Q, Chen C, Yao J. Vessel density and retinal thickness from optical coherence tomography angiography as new indexes in adolescent myopia. J Ophthalmol. 2021;2021:6069833. https://doi.org/10.1155/2021/6069833.
Leng Y, Tam EK, Falavarjani KG, Tsui I. Effect of age and myopia on retinal microvasculature. Ophthalm Surg Las Imag Retin. 2018;49:925–31.
Borrelli E, Lonngi M, Balasubramanian S, Tepelus TC, Baghdasaryan E, Iafe NA, et al. Macular microvascular networks in healthy pediatric subjects. Retina. 2019;39:1216–24.
Cheung CY, Li J, Yuan N, Lau GYL, Chan AYF, Lam A, et al. Quantitative retinal microvasculature in children using swept-source optical coherence tomography: the Hong Kong Children Eye Study. Br J Ophthalmol. 2018; https://doi.org/10.1136/bjophthalmol-2018-312413.
Zhang Y, Zhang B, Fan M, Gao X, Wen X, Li Z, et al. The vascular densities of the macula and optic disc in normal eyes from children by optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2020;258:437–44.
Kiziltoprak H, Tekin K, Cevik S, Kocer AM, Goker YS. Normative data assessment of peripapillary and macular vessel density and foveal avascular zone metrics using optical coherence tomography angiography in children. J Pediatr Ophthalmol Strabismus. 2020;57:388–98.
Zhang Z, Huang X, Meng X, Chen T, Gu Y, Wu Y, et al. In vivo assessment of macula in eyes of healthy children 8 to 16 years old using optical coherence tomography angiography. Sci Rep. 2017;7:8936.
Li S, Yang X, Li M, Sun L, Zhao X, Wang O, et al. Developmental changes in retinal microvasculature in children: a quantitative analysis using optical coherence tomography angiography. Am J Ophthalmol. 2020;219:231–9.
Hsu ST, Ngo HT, Stinnett SS, Cheung NL, House RJ, Kelly MP, et al. Assessment of macular microvasculature in healthy eyes of infants and children using OCT angiography. Ophthalmology. 2019;126:1703–11. https://doi.org/10.1016/j.ophtha.2019.06.028.
Sasongko MB, Wang JJ, Donaghue KC. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010;33:1331–6.
Savini G, Hoffer KJ, Schiano-Lomoriello D. Agreement between lens thickness measurements by ultrasound immersion biometry and optical biometry. J Cat Ref Surg. 2018;44:1463–8.
Author information
Authors and Affiliations
Contributions
AS, AGE, and AAM were responsible for the design of the study. AS, SAS, AGE, and DAG conducted the study. AS, AGE, and AAM analysed the data and interpreted the results. AS and AGE drafted the manuscript. DAG and AAM revised the manuscript. All authors read an approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This report was approved by Cairo University research ethics committee (Approval ID: MS-130-2021) and followed the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shuaib, A., Salem, S.A., Elnahry, A.G. et al. Correlation of the macular microvasculature to the axial length in pediatric patients with high axial refractive errors. Eye 38, 507–513 (2024). https://doi.org/10.1038/s41433-023-02712-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02712-8