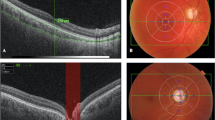
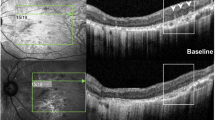
Abstract
Purpose
To assess the choroidal vascularity index (CVI) in patients affected by Leber hereditary optic neuropathy (LHON) compared to patients affected by dominant optic atrophy (DOA) and healthy subjects.
Methods
In this retrospective study, we considered three cohorts: LHON eyes (48), DOA eyes (48) and healthy subjects’ eyes (48). All patients underwent a complete ophthalmologic examination, including best-corrected visual acuity (BCVA) and optical coherence tomography (OCT) acquisition. OCT parameters as subfoveal choroidal thickness (Sub-F ChT), mean choroidal thickness (ChT), total choroidal area (TCA), luminal choroidal area (LCA) were calculated. CVI was obtained as the ratio of LCA and TCA.
Results
Subfoveal ChT in LHON patients did not show statistically significant differences compared to controls, while in DOA a reduction in choroidal thickness was observed (p = 0.344 and p = 0.045, respectively). Mean ChT was reduced in both LHON and DOA subjects, although this difference reached statistical significance only in DOA (p = 0.365 and p = 0.044, respectively). TCA showed no significant differences among the 3 cohorts (p = 0.832). No changes were detected in LCA among the cohorts (p = 0.389), as well as in the stromal choroidal area (SCA, p = 0.279). The CVI showed no differences among groups (p = 0.898): LHON group was characterized by a similar CVI in comparison to controls (p = 0.911) and DOA group (p = 0.818); the DOA group was characterized by a similar CVI in comparison to controls (p = 1.0).
Conclusion
CVI is preserved in DOA and LHON patients, suggesting that even in the chronic phase of the neuropathy the choroidal structure is not irreversibly compromised.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Theodorou-Kanakari A, Karampitianis S, Karageorgou V, Kampourelli E, Kapasakis E, Theodossiadis P, et al. Current and emerging treatment modalities for Leber’s hereditary optic neuropathy: A review of the literature. Adv Ther. 2018;35:1510–8.
Yu-Wai-Man P, Griffiths PG, Brown DT, Howell N, Turnbull DM, Chinnery PF. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 2003;72:333–9. Erratum in: Am J Hum Genet. 2016;98:1271.
Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–30.
Yu-Wai-Man P, Griffiths PG, Burke A, Sellar PW, Clarke MP, Gnanaraj L, et al. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology. 2010;117:1538–46.
Moster SJ, Moster ML, Bryan MS, Sergott RC. Retinal ganglion cell and inner plexiform layer loss correlate with visual acuity loss in LHON: A longitudinal, segmentation OCT analysis. Investig Ophthalmol Vis Sci. 2016. https://doi.org/10.1167/iovs.15-17328.
Asanad S, Tian JJ, Frousiakis S, Jiang JP, Kogachi K, Felix CM, et al. Optical coherence tomography of the retinal ganglion cell complex in Leber’s hereditary optic neuropathy and dominant optic atrophy. Curr Eye Res. 2019;44:638–44.
Barboni P, Carbonelli M, Savini G, Ramos Cdo V, Carta A, Berezovsky A, et al. Natural history of Leber’s hereditary optic neuropathy: Longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–7.
Borrelli E, Triolo G, Cascavilla ML, La Morgia C, Rizzo G, Savini G, et al. Changes in Choroidal Thickness follow the RNFL Changes in Leber’s Hereditary Optic Neuropathy. Sci Rep. 2016;6:37332.
Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2009. https://doi.org/10.1167/iovs.08-3325.
Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefe’s Arch Clin Exp Ophthalmol. 2011. https://doi.org/10.1007/s00417-011-1620-1.
Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090.
Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1901–8.
Weill Y, Brosh K, Levi Vineberg T, Arieli Y, Caspi A, Potter MJ, et al. Enhanced depth imaging in swept-source optical coherence tomography: Improving visibility of choroid and sclera, a masked study. Eur J Ophthalmol. 2020;30:1295–1300.
Park Y, Cho KJ. Choroidal vascular index in patients with open angle glaucoma and preperimetric glaucoma. PLoS One. 2019. https://doi.org/10.1371/journal.pone.0213336.
Velaga SB, Nittala MG, Vupparaboina KK, Jana S, Chhablani J, Haines J, et al. Choroidal vascularity index and choroidal thickness in eyes with reticular pseudodrusen. Retina. 2020;40:612–7.
Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36:1646–51.
Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–64.
Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014;55:3893–9.
Berenberg TL, Metelitsina TI, Madow B, Dai Y, Ying GS, Dupont JC, et al. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina. 2012;32:25–31.
Agrawal R, Ding J, Sen P, Rousselot A, Chan A, Nivison-Smith L, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retin Eye Res. 2020;77:100829.
Zhao M, Alonso-Caneiro D, Lee R, Cheong AMY, Yu WY, Wong HY, et al. Comparison of choroidal thickness measurements using semiautomated and manual segmentation methods. Optom Vis Sci. 2020;97:121–7.
Agrawal R, Seen S, Vaishnavi S, Vupparaboina KK, Goud A, Rasheed MA, et al. Choroidal vascularity index using swept-source and spectral-domain optical coherence tomography: A comparative study. Ophthalmic Surg Lasers Imaging Retin. 2019;50:e26–e32.
Giannaccare G, Pellegrini M, Sebastiani S, Bernabei F, Moscardelli F, Iovino C, et al. Choroidal vascularity index quantification in geographic atrophy using binarization of enhanced-depth imaging optical coherence tomographic scans. Retina. 2020;40:960–5.
Sacconi R, Battista M, Borrelli E, Senni C, Tombolini B, Grosso D, et al. Choroidal vascularity index is associated with geographic atrophy progression. Retina. 2022;42:381–7.
Kim M, Ha MJ, Choi SY, Park YH. Choroidal vascularity index in type-2 diabetes analyzed by swept-source optical coherence tomography. Sci Rep. 2018. https://doi.org/10.1038/s41598-017-18511-7.
Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012;119:2334–42.
Park JW, Suh MH, Agrawal R, Khandelwal N. Peripapillary choroidal vascularity index in glaucoma—A comparison between spectral-domain OCT and OCT angiography. Investig. Ophthalmol Vis Sci. 2018. https://doi.org/10.1167/iovs.18-24315.
Pellegrini M, Giannaccare G, Bernabei F, Moscardelli F, Schiavi C, Campos EC. Choroidal vascular changes in arteritic and nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2019;205:43–49.
Darvizeh F, Asanad S, Falavarjani KG, Wu J, Tian JJ, Bandello F, et al. Choroidal thickness and the retinal ganglion cell complex in chronic Leber’s hereditary optic neuropathy: A prospective study using swept-source optical coherence tomography. Eye (Lond) 2020;34:1624–30.
Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004. https://doi.org/10.1016/j.preteyeres.2003.10.003.
Chevrollier A, Guillet V, Loiseau D, Gueguen N, de Crescenzo MA, Verny C, et al. Hereditary optic neuropathies share a common mitochondrial coupling defect. Ann Neurol. 2008;63:794–8.
Sadun A, Carelli V, La Morgia C, Karanjia R. Leber’s Hereditary Optic Neuropathy (LHON) mtDNA mutations cause cell death by overproduction of reactive oxygen species. Acta Ophthalmol. 2015. https://doi.org/10.1111/j.1755-3768.2015.0131.
Borrelli E, Balasubramanian S, Triolo G, Barboni P, Sadda SR, Sadun AA. Topographic macular microvascular changes and correlation with visual loss in chronic leber hereditary optic neuropathy. Am J Ophthalmol. 2018;192:217–28.
Acknowledgements
VC reports consultant and Advisory Board activities with GenSight Biologics, Pretzel Therapeutics, Stealth Biotherapeutics and Chiesi Farmaceutici; honoraria from Chiesi Farmaceutici, First Class and Medscape. None of these activities are related to conduction of this study and the writing of the manuscript. FB is Consultant for Allergan, Bayer, Boehringer-Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Thrombogenics, Zeiss. None of these activities are related to conduction of this study and the writing of the manuscript. PB reports consultancies for GenSight Biologics and received speaker honoraria from Santhera Pharmaceuticals, Chiesi Farmaceutici, Omikron Italia; he is SI for clinical trials sponsored by GenSight Biologics and Santhera. None of these activities are related to conduction of this study and the writing of the manuscript. All other authors declare no financial disclosures.
Author information
Authors and Affiliations
Contributions
MB, MLC, and PB were responsible for the ideation, design and conduction of the study. CB, GL, CV, and AB acquired the data and the measurements for the analysis. EB performed the statistical analysis. LC and VC counselled for the genetic. MB, EB, and PB critically reviewed the results obtained. MB, EB, and PB contributed to the writing of the paper. MLC and VC performed the last revision of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battista, M., Cascavilla, M.L., Borrelli, E. et al. Choroidal vascularity index in hereditary optic neuropathies. Eye 37, 2679–2684 (2023). https://doi.org/10.1038/s41433-023-02383-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02383-5