Abstract
Background
To test the hypothesis that elevated plasma levels of homocysteine (Hcy) and lipoprotein (a) (LPA) contribute to diabetic retinopathy (DR) associated with dysregulated lipid profile, dyslipidaemia, and kidney function.
Methods
A total of 83 patients with type 2 diabetes mellitus (T2DM) were enrolled in this prospective case-control study. Patients were categorized into those with no DR (DM), non-proliferative DR (NPDR), and proliferative DR (PDR). Age and sex-matched individuals with no diabetes were included in the control group. Biochemical tests, including fasting blood glucose (FBG), glycated hemoglobin (HbA1c), Hcy, LPA, lipid profile, and urine microalbumin (UMA), were evaluated.
Results
Hcy was negatively correlated with high-density lipoprotein-cholesterol (HDL-C) (p < 0.05), but positively correlated with [total cholesterol (TC)-HDL-C)/HDL-C] (p < 0.05), low-density lipoprotein cholesterol (LDL-C)/HDL-C (p < 0.05), and UMA (p < 0.05). Traditional risk factors, Hcy, arteriosclerosis-associated plasma indices, and UMA, resulted as the independent risk factors for the occurrence of DM and DR. After controlling for age, sex, duration of DM, and FBG, a multiple ordinal logistic regression model showed that LPA [OR = 2.90, 95% confidence interval (95% CI) 1.16–7.23, p = 0.023)], LDL-C (OR = 4.28, 95% CI 1.24–14.79, p = 0.021), and (TC-HDL-C)/HDL-C (OR = 1.92, 95% CI 1.05–3.53, p = 0.035) were risk factors for DM and DR.
Conclusions
Hcy and LPA contributed to DM and DR. Hcy was positively correlated with kidney dysfunction and the ratios of lipid profiles, and negatively with HDL-C, LPA, LDL-C, and (TC-HDL-C)/HDL-C resulted as predictors of the occurrence of DM and severity of DR.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is one of the most severe microvascular complications of diabetes mellitus (DM) and a leading cause of visual impairment among individuals aged 20–74 years [1]. Traditional strong risk factors of DR include duration of diabetes, fasting blood glucose (FBG), and systemic hypertension, while a weaker association exists with glycated hemoglobin (HbA1c) and lipid profile, including low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC). However, the landmark cohort studies on people with type 2 diabetes (T2DM), including the UK Prospective Diabetes Study (UKPDS), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, and the Veteran Affairs Diabetes Trial (VADT) revealed that after follow-up for 3–13 years, the risk of occurrence of diabetic kidney disease (DKD, 25%) and DR (51%) in patients with T2DM remained high despite strict control of hyperglycemia, hypertension, and hyperlipidaemia [2]. The results from these large cohort studies may be explained by the fact that microvascular damage may have already occurred before control of hyperglycemia, hypertension, and hyperlipidaemia and/or other non-traditional circulating risk factors may also contribute to DR.
Homocysteine (Hcy) and lipoprotein (a) (LPA) have been identified as important contributors to diabetic nephropathy (DN) and cardiovascular disease [3]. Hcy is a sulfur-containing amino acid produced when proteins are broken down. Hcy releases free apolipoprotein (a) (apoA) in plasma and discloses its full antifibrinolytic activity partially hidden in the LPA particle by reducing the apoA/apoB-100 apoB disulfide bond [4]. Existing studies have reported on the correlation between Hcy and LPA in macro and micro-vascular diseases, including stroke, cardiovascular diseases [5], diabetes and its microvascular complications; yet little is known about their associations with T2DM and DR. We have previously shown that lipid profile, especially the arteriosclerosis-associated plasma indices LDL-C/high-density lipoprotein-cholesterol (HDL-C), TC-(HDL-C)/HDL-C, and triglycerides (TG)/HDL-C are independent risk factors for DR [6]. However, the regulatory mechanisms of these traditional lipid parameters remain uncertain, and the correlations between Hcy, LPA, and the lipid profiles warrant further investigation.
The aim of the present study is to evaluate the possible role of non-traditional risk factors such as Hcy and LPA in the occurrence of T2DM and DR and their associations with other traditional DR risk factors, especially the arteriosclerosis-associated plasma indices.
Methods
Participants
This prospective case-control study was approved by Beijing Tongren Hospital, Capital Medical University, and followed the Ethical Principles for Medical Research of the Declaration of Helsinki. Written informed consent was obtained from all subjects before they were enrolled in the study. Patients were assigned to the DM group, non-proliferative DR (NPDR) group, and proliferative DR (PDR) group [6]. Inclusion criteria included: T2DM was diagnosed according to the 2020 American Diabetes Association (ADA) guidelines of DM; the classification and diagnostic criteria of DR were in accordance with the 2017 ADA “A Position Statement of DR” [7, 8]; participants with DM but without DR in both eyes were assigned into the “DM” group; those without neovascularization in either eye were assigned to the NPDR group; participants were assigned into the “PDR” group if retinal and/or optic disc neovascularization with or without iris/angle neovascularization was found in at least one eye (neovascularization was confirmed by slit lamp, fundus photography and optical coherence tomography angiography).
Exclusion criteria included: (1) participants with other retinal diseases such as non-diabetic retinal vascular disease, age-related macular degeneration, uveitis, and inherited retinal diseases (2) posterior segment or cataract surgery within 6 months (3) ocular media opacity and/or inability to tolerate examinations due to severe system diseases; (4) patients with a history of lipid disorders or on lipid-lowering therapy.
Age- and sex-matched control subjects were enrolled at the same time as other patients. The study subjects, including the patients with DM, NPDR, and PDR, and normal controls, were selected from our Type 2 Diabetes Mellitus and Diabetic Retinopathy Study Cohort, a prospective cohort established from 2016 to 2022 in Beijing Tongren Eye Centre.
Data collection, eye examination, and biochemical test
Baseline information such as age, gender, medical history (DM and hypertension), and medications (insulin, lipid-lowering therapy) were collected. Considering that metformin usage may be correlated with the vitamin B12 (vitB12) deficiency, thus inducing elevated serum level of Hcy, we collected the related information, including the dosage and duration of metformin intake, vitB12, and/or folic acid supplement from each enrolled patient.
The participants underwent an assessment of best-corrected visual acuity (BCVA), non-contact intraocular pressure (TX20 Automatic Non-contact Tonometer, Canon Co., Ltd, Tokyo, Japan), slit-lamp microscopic examination (SL-IE Slit Lamp Microscope, Topcon Co., Ltd, Tokyo, Japan), and fundus examination with mydriasis (with tropicamide eye drops, Santen Pharmaceutical Co., Ltd.). Fundus photography (CR-1 Fundus Camera, Canon Co., Ltd.) was applied to capture at least two-field centered on the optic disc and macula of both eyes. Two independent ophthalmologists ascertained the DR status based on the International DR severity scale [8]. Swept-source optical coherence tomography with 9 × 9 mm scanning range mode was applied (DRI OCT1 Atlantis scanner, Topcon Co., Ltd., Tokyo, Japan or Plex Elite 9000, Carl Zeiss Meditec, Inc, Oberkochen, German) to obtain B-scan mode for all the enrolled subjects as previously described [6].
Blood biochemistry profile, including Hcy, LPA, TC, TG, LDL-C, HDL-C, HbA1c and FBG, was estimated. The plasma level of Hcy was measured using the enzymatic cycling method (Beckman AU5821; Leadman determination kit). Urinary microalbumin (UMA) was also tested for all the enrolled subjects. UMA concentration was measured using immunonephelometry (Beckman AU5811; inter-assay CV 3–5%).
Definition of dyslipidaemia was based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel-III (ATP-III) report in 2001 [9]. Dyslipidaemia was defined as elevated TC, TG or LDL-C (TC > 5.17 mmol/L, TG > 1.70 mmol/L, or LDL-C > 3.37 mmol/L).
Definition of hyperhomocysteinemia and abnormal LPA level: Hcy >10 μmol/L was defined as hyperhomocysteinemia according to the 2006 guidelines for the Prevention of Stroke from the American Heart Association and American Stroke Association [10]. LPA > 30 mg/dl was defined as an abnormal LPA level according to 2016 Canadian guidelines for Dyslipidaemia Management and Guidelines for the Prevention [11].
Determination of the cut-off value of arteriosclerosis indices
Arteriosclerosis indices including API (LDL-C/HDL-C), AI [(TC-HDL-C)/HDL-C], and AIP [log (TG/HDL-C)] were calculated. As we previously described [6], the cut-off values were determined by receiver operating characteristic curves. Patients with API > 2.24 [area under curve (AUC): 0.746; sensitivity = 0.708, specificity = 0.517), AI > 2.91 (AUC: 0.723; sensitivity = 0.629; specificity = 0.724) or AIP > 0.01 (AUC: 0.564; sensitivity = 0.607, specificity = 0.552) were assigned to high API, high AI, and high AIP groups, respectively [6].
Sample size calculation
The sample size was determined using the Power Analysis and Sample Size (PASS) 15 software. According to our pilot study, the mean level of Hcy in the study and control groups was 14.7 μmol/L and 11.5 μmol/L, respectively (SD: ± 3). The minimum number per arm (sample size) was 20 people to detect the difference between the four groups with the designed power (1- beta = 90%) at a 95% confidence level (alpha = 0.05), as we previously described [6].
Statistical analysis
SPSS software (SPSS, Inc. 25.0, Chicago, IL, USA) was used for all statistical analyses. Normality was assessed by the Shapiro–Wilk test. Homogeneity of variance was tested by the Levene test. Age, duration of DM, Hcy, LPA, HbA1c, TG, TC, LDL-C, HDL-C, atherogenic index (AI, TC-HDL-C/HDL-C), atherogenic index of plasma [AIP, log (TG/HDL-C)] and atherogenic plasma index (API, LDL-C/HDL-C) levels in each group were described by mean (standard deviation) or median (interquartile range) according to characteristics. Kruskal–Wallis H test and one-way ANOVA were used for comparison between groups. A Chi-square test was used to compare the gender and duration of hypertension (HBP) in each group. Spearman’s rank correlation coefficient analysis was used to test the correlations of Hcy and LPA with lipid profiles and UMA in Control, DM, NPDR, and PDR groups. Multiple logistic regression was used to analyze the effect of gender, duration of DM, duration of HBP, Hcy, LPA, HbA1c, and other factors in control subjects, patients with DM, and DR. Bonferroni corrections were applied for the groups’ comparison between the four groups. p < 0.05 indicated statistical significance.
Results
Baseline demographic and clinical characteristics
A total of 83 patients with T2DM were assigned to the DM group [28 cases, aged 42–75 years, (58.14 ± 9.18) years], NPDR group [25 cases, aged 29–76 years, (54.08 ± 10.93) years], and PDR group [30 cases, aged 27–73 years, (54.00 ± 9.22) years]. Twenty-eight age- and sex-matched healthy subjects aged 40–74 years (55.61 ± 8.95) were enrolled as the controls.
There was no significant difference in age and gender among the four groups (page = 0.335, p gender = 0.677). The duration of DM significantly differed in the DM, NPDR, and PDR groups (p < 0.001). There were significant differences in Hcy (H = 8.02, p = 0.04), FBG (H = 51.58, p < 0.001), HbA1c (p < 0.001), TC (H = 7.97, p = 0.047), LDL-C (H = 10.07, p = 0.018), HDL-C (H = 8.93, p = 0.030), UMA (H = 21.85, p < 0.001), AI (H = 10.90, p = 0.012) and API (H = 11.00, p = 0. 012) between these groups. No significant difference was found in LPA, TG, and AIP among the four groups, as shown in Table 1.
Hcy level was significantly higher in control male subjects than in female ones [12.05 (10.23–15.48) μmol/L vs. 9.35 (8.68–10.00) μmol/L, p = 0.007]. Similarly, a significantly higher level of Hcy was found in male than in female patients with type2 DM (no DR) [14.03 ± 3.78 μmol/L vs. 12.08 ± 3.39 μmol/L, p = 0.023]; however, there was no gender difference in the NPDR or PDR groups (Table 1).
Correlations of Hcy and LPA with lipid profiles and UMA in Control, DM, NPDR, and PDR groups
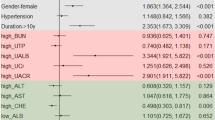
Spearman’s rank correlation coefficient analysis showed that Hcy was significantly positively correlated with AI (r = 0.23, p < 0.05), API (r = 0.20, p < 0.05), and UMA (r = 0.22, p < 0.05) but negatively correlated with HDL-C (r = −0.21, p < 0.05). Hcy was also positively correlated with TC, TG, LDL-C, and AIP, but the statistical difference was not significant. LPA was found to be positively correlated with LDL-C, AI, and API (p > 0.05) and negatively correlated with TC, TG, HDL-C, AI, and UMA (p > 0.05), but there was no statistical significance (Fig. 1).
Spearman’s rank correlation coefficient analysis showed that Hcy was significantly positively correlated with AI, API, and UMA but negatively correlated with HDL-C. *Statistically significant: p < 0.05. Hcy Homocysteine, LPA lipoprotein (a), TC total cholesterol, TG triglycerides, LDL-C Low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol, AI Atherosclerosis index [(TC-HDL-C)/HDL-C], AIP Atherogenic index of plasma [log (TG/HDL-C)], API Atherogenic plasma index (LDL-C/HDL-C), UMA Urinary microalbumin.
Effects of metformin usage and vitB12 or vitB12 and folic acid supplement on serum level of Hcy
There was no statistical significance in the serum level of Hcy between the no metformin usage without vitB12 or folic acid supplement (15.17 ± 8.12 μmol/L), metformin usage without vitB12 use (16.38 ± 5.85 μmol/L), no metformin usage with vitB12 supplement (10.94 ± 2.55 μmol/L) and metformin use with vitB12 supplement (11.56 ± 3.92 μmol/L) groups (p = 0.101), although lower serum level of Hcy was found in those subjects with metformin usage without vitB12 supplement in comparison with the vitB12 intake (16.38 ± 5.85 vs. 11.56 ± 3.92 μmol/L) (Table 2).
Effects of Hcy, LPA, lipid profiles, and other traditional risk factors in Control, DM and NPDR, and PDR groups
When Control, DM, NPDR and PDR groups were input as continuous independent variables in univariate ordinal logistic regression model, duration of DM (OR = 1.20, 95% CI 1.13–1.27, p < 0.001) and HBP duration (OR = 2.73, 95% CI 1.35–5.52, p = 0.005), HbA1c (OR = 2.61, 95% CI 1.96–3.49, p < 0.001) and FBG (OR = 1.71, 95% CI 1.42–2.05, p < 0.001) resulted as independent risk factors affecting the occurrence and severity of DM and DR. When Hcy (Hcy < 10 μmol/L) were considered as categorical variable, TC (OR = 1.47, 95% CI 1.03–2.11, p = 0.035), LDL-C (OR = 1.55, 95% CI 1.03–2.33, p = 0.034), AI (OR = 1.58, 95% CI 1.19–2.09, p = 0.001), API (OR = 1.80, 95% CI 1.24–2.61, p = 0.002), UMA (OR = 1.04, 95% CI 1.01–1.08, p = 0.025) and Hcy group (OR = 2.78, 95% CI 1.25–6.20, p = 0.013) resulted as strong risk factors affecting the occurrence and severity of DM and DR (Table 3).
Multiple ordinal logistic regression models showed LPA and LDL-C were strong risk factors for the occurrence of DM and DR
When Control, DM, NPDR and PDR groups were considered as continuous independent variables, after controlling for age and sex, duration of DM (OR = 1.20, 95% CI 1.11–1.29, p < 0.001), FBG (OR = 1.30, 95% CI 1.02–1.67, p = 0.035), LPA group (OR = 2.90, 95% CI 1.16–7.23, p = 0.023), LDL-C (OR = 4.28, 95% CI 1.24–14.79, p = 0.021) and AI (OR = 1.92, 95% CI 1.05–3.53, p = 0.035) resulted as strong risk factors for DM and DR (Fig. 2).
Multivariable ordered logistic model showing LPA contributes to DM and DR associated with DM duration, FBG, LDL-C, and AI. *Statistically significant: p <0.05. DM Diabetes mellitus, HBP High blood pressure, HbA1c Glycated hemoglobin, FBG Fasting blood glucose, Hcy Homocysteine, LPA lipoprotein (a), TC total cholesterol, LDL-C Low-density lipoprotein cholesterol, AI Atherosclerosis index [(TC-HDL-C)/HDL-C], AIP Atherogenic index of plasma [log (TG/HDL-C)], API Atherogenic plasma index (LDL-C/HDL-C), UMA Urinary microalbumin.
Discussion
Our data suggest that Hcy and LPA contribute to the presence of DM and DR. Hcy was positively correlated with AI, API, and UMA and negatively correlated with HDL-C in DR. Univariate logistic regression model further showed that Hcy was significantly associated with DM and DR. Though no significant associations were found in multiple logistic regression model, we believe that Hcy may indirectly contribute to DR through its effects on AI, API, UMA and HDL-C.
The univariate logistic regression model revealed that Hcy was significantly higher in DM and DR groups than that in the control group, indicating that Hcy may contribute to DR, which is consistent with previous findings [12]. The existing evidence can explain the possible mechanism of the contribution of Hcy on DR. Hcy can induce the expression of CD 11b/ CD 18 (a member of the leukocyte integrin family of heterodimeric adhesion molecules) to form a docking complex, exacerbating the inflammation response of the endothelium [13]. This pathological process further stimulates the proliferation of smooth muscle cells and promotes the peroxidation of LDL-C, resulting in thrombosis activation and vascular dysfunction [14]. In addition, Hcy contributes to the breakdown of the blood-retinal barrier (BRB) and induces retinal ischemia and angiogenesis by increasing the level of retinal vascular endothelial growth factor (VEGF) [15]. It also induces retinal ganglion cell death [16]. Furthermore, Hcy downregulates HDL-C by inhibiting apoA-1, which is associated with enhancing HDL-C clearance [17, 18]. Moreover, Hcy is poorly removed in patients with DR due to impaired sulfidation and demethylation. However, in our study, after controlling for age and gender, multiple ordinal logistic regression showed that LPA, AI, and LDL-C were predictors for DM and DR, indicating that Hcy contributes to the occurrence and progression of DM and DR through its interactions with AI, API, and UMA. As this may also be due to the limited sample size in each group; a large cohort study is warranted to further validate the current study result.
Our results also showed that a higher level of Hcy was significantly negatively correlated with HDL-C, which was consistent with previous studies on cardiovascular and atherosclerotic diseases [19, 20]. The molecular mechanisms underlying homocysteine’s role in HDL metabolism have not yet been fully elucidated; however, some studies provided interesting insights. In a cystathionine β-synthase, +/+ mice model (high-level Hcy), impaired HDL cholesterol acyltransferase function with elevated Hcy levels was observed and was accompanied by increased HDL cholesterol ester clearance due to the up-regulated expression level of scavenger receptor B-I (SR-B1) in the liver [17]. As HDL-C has been considered a protective factor for DR, Hcy may also indirectly contribute to DR via down-regulated HDL-C. Moreover, the negative correlation between Hcy and HDL-C was also found in patients with myocardial infarction and atherosclerosis, suggesting that Hcy contributes to micro- or macrovascular diseases through downregulation of serum level of HDL-C [19].
In the present study, Hcy and UMA were positively correlated and resulted as risk factors for the occurrence and development of DR, which is consistent with previous studies [21]. High-level Hcy has been reported as an independent risk factor for renal injury in patients with DN, as Hcy is mainly synthesized and metabolized in the kidney [22]. Decreased glomerular filtration rate (GFR) is correlated with increased serum level of Hcy in early renal tubular epithelial diseases. This potentially occurs through high-level Hcy, which causes microvascular thrombosis, dysfunction of endothelial cells, and reduces glomerular filtration by activating oxidative stress and nitric oxide pathways. This process further induces direct endothelial cells cytotoxicity and up-regulates coagulation factors [21]. We concluded that Hcy and UMA are predictors of DR progress, and Hcy may also be a link between DR and DN.
We found that plasma level of Hcy was significantly higher in male than in female subjects, either in the control group or in patients with T2DM, which provided further support for the premise that males in general, and especially those with diabetes, suffer from a higher degree of atherosclerosis across all age groups [23]. Furthermore, although the gender differences in Hcy are still not clear, it is possible that they occur due to the higher plasma concentrations of apoA-1 and HDL-C found in women compared to men, and TG and uric acid, which are lower in women than in men. These observations suggest that Hcy has complicated interactions with lipid profiles [24].
The influence of metformin usage and vitB12 supplement on Hcy was omitted in this study. Metformin is the first therapeutic choice for T2DM management. Numerous observational and interventional studies have shown that an average of 6–30% of patients with varying degrees of vitB12 deficiency and subsequent increase in the serum level of Hcy caused by metformin usage were inversely dependent on its dose and duration [25]. Still, a conclusive link between metformin usage and increased serum level of Hcy remains elusive. Only 10 out of 17 randomized trials reported that vitB12 deficiency was correlated with metformin use by a systemic review [26]. A previous cross-sectional study found no statistical significance in the vitB12 serum level between the metformin and non-metformin users with T2DM in Qatar [27]. In the current study, we found no significant difference between the four groups (metformin use with vitB12 supplement, metformin usage without vitB12 use, no metformin usage with vitB12 supplement, and no metformin use without vitB12 supplement), which is consistent with a previous study [26].
In this study, serum level of Hcy ≥10 μmol/L was defined as hyperhomocysteinemia according to the 2006 guidelines for Prevention of Stroke by the American Heart Association and American Stroke Association [10]. Increasing evidence has shown that 10 μmol/L of Hcy in the serum is an independent risk factor for cognitive impairment [28], Alzheimer’s disease [28], peripheral arterial disease [29], H-Type hypertension [30], stroke [30], cardiovascular diseases [31] and endothelial dysfunction [32]. As a dysfunction of the neurovascular unit is recognized as the pathological basis for both stroke and retinal vascular diseases, there is a similarity in the dynamic neurovascular unit coupling between the brain and retina; in this study, we followed the diagnostic criteria of 10 μmol/L for stroke.
Multiple ordinal logistic regression models also showed that LPA was a strong independent risk factor for DM and DR. LPA is low-density lipoprotein (LDL)-like substance related to heritability. Thus far, no effective treatment has been found to lower the plasma LPA concentration in humans [33]. LPA has been considered a link between atherosclerosis and thrombosis and DM, as it shares a high degree of sequence homology with plasminogen [34]. Controversially, some studies also showed that LPA was not correlated with diabetes [35]. Similarly, in the present study, LPA was not statistically significant in univariate ordinal logistic regression; however, this might be due to the limited sample size.
In the present study, the correlations between Hcy and LPA were not statistically significant. Some studies have shown that Hcy increased the affinity of LPA to fibrin, leading to LPA-mediated impairment of fibrinolysis and arteriosclerosis. Moreover, high homocysteine and LPA synergistically increased the incidence of coronary heart disease and had a synergistic role in diabetic foot and peripheral artery disease [36, 37]. All the above evidence suggests that Hcy and LPA may have a synergistic effect on DR. A prospective cohort study with larger sample size is needed to further explore the synergy effects of Hcy and LPA in DR.
In conclusion, we found that Hcy and LPA contributed to DM and DR. Hcy was positively correlated with AI, API, and UMA and negatively correlated with HDL-C. Also, AI and LDL-C resulted as predictors of the presence and severity of DR. In addition, some non-traditional risk factors such as Hcy and LPA should also be considered in the prevention and treatment of DR.
Summary
What was known before
-
Homocysteine (Hcy) and lipoprotein (a)(LPA) contributed to type 2 diabetes and diabetic retinopathy.
-
Hcy was positively correlated with [(total cholesterol(TC)-high-density lipoprotein cholesterol(HDL-C)/HDL-C], low-density lipoprotein cholesterol(LDL-C)/HDL-C, urine microalbumin and negatively correlated with HDL-C, (TC-HDL-C)/HDL-C and LDL-C resulted as predictors of the presence and severity of DR.
What this study adds
-
Some non-traditional risk factors such as Homocysteine and lipoprotein (a) should also be considered in the prevention and treatment of DR.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
van Reyk DM, Gillies MC, Davies MJ. The retina: oxidative stress and diabetes. Redox Rep. 2003;8:187–92.
Zoungas S. Advance in context: The benefits, risks and feasibility of providing intensive glycaemic control based on gliclazide modified release. Diabetes Obes Metab. 2020;22:5–11.
Chico A, Pérez A, Córdoba A, Arcelús R, Carreras G, de Leiva A, et al. Plasma homocysteine is related to albumin excretion rate in patients with diabetes mellitus: a new link between diabetic nephropathy and cardiovascular disease? Diabetologia. 1998;41:684–93.
Nardulli M, Durlach V, Pepe G, Anglés-Cano E. Mechanism for the homocysteine-enhanced antifibrinolytic potential of lipoprotein(a) in human plasma. Thromb Haemost. 2005;94:75–81.
Zhang L, Li Z, Xing C, Ma X, Xu R. The protective mechanism of folic acid on hyperhomocysteinemia-related arterial injury in spontaneously hypertensive rats: Folic acid against arterial inflammation. Vascular. 2021. https://doi.org/10.1177/17085381211036549.
Zhang XY, Qiu BJ, Wang QY, Sivaprasad S, Wang YH, Zhao L, et al. Dysregulated serum lipid metabolism promotes the occurrence and development of diabetic retinopathy associated with upregulated circulating levels of VEGF-A, VEGF-D, and PlGF. Front Med. 2021. https://doi.org/10.3389/fmed.2021.779413.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9.
Bressler SB, Ayala AR, Bressler NM, Melia M, Qin H, Ferris FL 3rd, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134:278–85.
National cholesterol education program (NCEP) expert panel on detection e, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–449.
Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–82.
Brazionis L, Rowley K, Itsiopoulos C, Harper CA, O’Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31:50–56.
Pushpakumar S, Kundu S, Sen U. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Curr Med Chem. 2014;21:3662–72.
Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11:56–64.
Yan TT, Li Q, Zhang XH, Wu WK, Sun J, Li L, et al. Homocysteine impaired endothelial function through compromised vascular endothelial growth factor/Akt/endothelial nitric oxide synthase signalling. Clin Exp Pharm Physiol. 2010;37:1071–7.
Moore P, El-sherbeny A, Roon P, Schoenlein PV, Ganapathy V, Smith SB. Apoptotic cell death in the mouse retinal ganglion cell layer is induced in vivo by the excitatory amino acid homocysteine. Exp Eye Res. 2001;73:45–57.
Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006;99:598–606.
Devlin AM, Lentz SR. ApoA-I: a missing link between homocysteine and lipid metabolism? Circ Res. 2006;98:431–3.
Qujeq D, Omran TS, Hosini L. Correlation between total homocysteine, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in the serum of patients with myocardial infarction. Clin Biochem. 2001;34:97–101.
Momin M, Jia J, Fan F, Li J, Dou J, Chen D, et al. Relationship between plasma homocysteine level and lipid profiles in a community-based Chinese population. Lipids Health Dis. 2017;16:54.
Wang T, Wang Q, Wang Z, Xiao Z, Liu L. Diagnostic value of the combined measurement of serum hcy, serum cys C, and urinary microalbumin in type 2 diabetes mellitus with early complicating diabetic nephropathy. ISRN Endocrinol. 2013. https://doi.org/10.1155/2013/407452.
Zoccali C, Jager KJ. Hyperhomocysteinemia: a renal and cardiovascular risk factor? Nat Rev Nephrol. 2010;6:695–6.
Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol. 2014;8:49–59.
Abdella NA, Mojiminiyi OA, Akanji AO, Moussa MA. Associations of plasma homocysteine concentration in subjects with type 2 diabetes mellitus. Acta Diabetol. 2002;39:183–90.
Kim J, Ahn CW, Fang S, Lee HS, Park JS. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine. 2019. https://doi.org/10.1097/md.0000000000017918.
Chapman LE, Darling AL, Brown JE. Association between metformin and vitamin B(12) deficiency in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2016;42:316–27.
Elhadd T, Ponirakis G, Dabbous Z, Siddique M, Chinnaiyan S, Malik RA. Metformin use is not associated with B(12) deficiency or neuropathy in patients with type 2 diabetes mellitus in Qatar. Front Endocrinol. 2018;9:248.
Lauriola M, D’Onofrio G, Ciccone F, Germano C, Cascavilla L, Paris F, et al. Relationship of homocysteine plasma levels with mild cognitive impairment, alzheimer’s disease, vascular dementia, psychobehavioral, and functional complications. J Alzheimers Dis. 2021;82:235–48.
Liu M, Fan F, Liu B, Jia J, Jiang Y, Sun P, et al. Joint effects of plasma homocysteine concentration and traditional cardiovascular risk factors on the risk of new-onset peripheral arterial disease. Diabete Metab Synd Ob. 2020;13:3383–93.
Qin X, Huo Y. H-Type hypertension, stroke and diabetes in China: opportunities for primary prevention. J Diabetes. 2016;8:38–40.
Chori BS, Danladi B, Inyang BA, Okoh MP, Nwegbu MM, Alli AL, et al. Hyperhomocysteinemia and its relations to conventional risk factors for cardiovascular diseases in adult nigerians: the REMAH study. BMC Cardiovasc Disor. 2021;21:102.
de Groot PG, Willems C, Boers GH, Gonsalves MD, van Aken WG, van Mourik JA. Endothelial cell dysfunction in homocystinuria. Eur J Clin Invest. 1983;13:405–10.
Kostner KM, März W, Kostner GM. When should we measure lipoprotein (a)? Eur Heart J. 2013;34:3268–76.
Chopra R, Saramma JG, Mary J, Rebecca A. Lipoprotein(a) as a risk factor for diabetic retinopathy in patients with type 2 diabetes mellitus. Indian J Ophthalmol. 2007;55:195–8.
Tu WJ, Liu H, Liu Q, Cao JL, Guo M. Association between serum lipoprotein(a) and diabetic retinopathy in han Chinese patients with type 2. diabetes J Clin Endocr Metab. 2017;102:2525–32.
Chandni R, Ramamoorthy KP. Lipoprotein(a) in type 2 diabetic subjects and its relationship to diabetic microvascular complications. World J Diabetes. 2012;3:105–9.
Gazzaruso C, Coppola A, Montalcini T, Baffero E, Garzaniti A, Pelissero G, et al. Lipoprotein(a) and homocysteine as genetic risk factors for vascular and neuropathic diabetic foot in type 2 diabetes mellitus. Endocrine. 2012;41:89–95.
Funding
This work was supported by the National Natural Science Foundation of China [Grant 81570850 and 82070988] and the Ministry of Science and Technology Foundation of China [Grant 2016YFC1305604].
Author information
Authors and Affiliations
Contributions
XZ contributed to conception and design of the study, drafted and revised the manuscript and perform statistical analysis. XC and YN organized the database, performed the experiments and statistical analysis and drafted the manuscript. ZG, AF and XC performed the statistical analysis. QW, BQ, RX and YW helped to enroll patients. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Zhang, X., Nie, Y. et al. Circulating level of homocysteine contributes to diabetic retinopathy associated with dysregulated lipid profile and impaired kidney function in patients with type 2 diabetes mellitus. Eye 37, 1383–1389 (2023). https://doi.org/10.1038/s41433-022-02144-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02144-w