Abstract
Aim/purpose
Bloodstream candida infections can seed the eye via hematogenous spread and result in chorioretinitis or endophthalmitis. If undetected and untreated, this can result in permanent vision loss. Past studies evaluating incidence of ocular candidiasis among hospitalized patients with positive fungal blood cultures have demonstrated variable rates of occurrence, but recent studies have generally shown a lower incidence than was reported several decades ago. Given low rates of occurrence, the utility of screening patients with dilated fundus exams has been called into question. The primary aim of this investigation is to identify the rate of chorioretinitis and endophthalmitis based on dilated fundoscopy for patients with fungemia at a tertiary care hospital.
Methods
This study was a retrospective chart review of adult patients admitted to the medical centre of the University of Arkansas for Medical Sciences (UAMS) between May 1, 2014 and December 31, 2017, who had positive fungal blood cultures during their hospitalization.
Results
There were 324 positive fungal cultures in 290 patients. Of this initial group, there were 161 eye exams. Ocular examination identified 7 of 161 patients (4.3%) with chorioretinitis or endophthalmitis.
Discussion
These outcomes along with previous studies support the current guidelines that screening with dilated fundus examination for these patients is appropriate and necessary.
Similar content being viewed by others
Introduction
Disseminated fungal infections are associated with high mortality and morbidity, with potential of hematogenous spread to the eye resulting in fungal chorioretinitis or endophthalmitis. While ocular fungal infections among hospitalized patients are uncommon, they can cause severe vision loss if not diagnosed and treated quickly. Older studies evaluating rates of ocular infection in patients with fungemia reported involvement in 10–45% of cases [1, 2]. More recent studies have suggested that the rate of endogenous fungal endophthalmitis and chorioretinitis is much lower, with rates <5% commonly being reported [1, 3,4,5,6,7]. This trend has been attributed to many factors including improvement in antifungal therapy, prompt initiation of treatment, as well as prophylactic treatment when clinical suspicion is high [8]. Despite this encouraging trend, recommendations to complete a screening eye exam for patients with fungemia have remained unchanged.
Current guidelines from the Infectious Disease Society of America (IDSA) for management of candidemia recommend a dilated fundoscopic examination by an ophthalmologist within the first week of diagnosis [9]. There are concerns about the cost effectiveness of ophthalmologic consultation in all patients with candidemia considering the low prevalence of ocular involvement and some argue that not all patients require screening [10, 11]. The IDSA maintains its position citing the potential for vision loss in patients with undetected fungal endophthalmitis. Articles advocating for continued use of screening exams cite higher rates of chorioretinitis in patients compared to those that question utility of such exams [12, 13]. With healthcare costs rising across the country, finding a more cost-effective way of screening for chorioretinitis could lead to a reduction in unnecessary costs. This could be accomplished by identifying risk factors for development of intraocular infection and focusing screening exams on those patients.
In patients with candidemia without metastatic complications, the IDSA recommends continuation of antifungal therapy for 2 weeks after documented clearance of Candida species from the bloodstream. In cases with ocular involvement, treatment should extend at least 4–6 weeks post clearance, with timing of medication cessation determined by repeated fundoscopic examination [9]. In cases of endophthalmitis, intravitreal antifungal therapy is also required. While discussion is ongoing about the necessity of ophthalmic examination for patients with candidemia, this practice is generally accepted as a useful screening tool for early detection of chorioretinitis and endophthalmitis. The aim of this study is to retrospectively identify the rate of chorioretinitis and endophthalmitis in a tertiary hospital based on dilated fundoscopy for patients with fungemia.
Methods
This study was a retrospective chart review of adult patients (≥18 years) admitted to the medical centre of the University of Arkansas for Medical Sciences (UAMS) between May 1, 2014 and December 31, 2017, who had positive fungal blood cultures during their hospitalization. The study that was approved by the UAMS Institutional Review Board was conducted in accordance with the tenets of the Declaration of Helsinki. Patients were identified by searching the hospital database for blood cultures that were positive for fungal species. Duplicate positive blood cultures on the same patient were included if they occurred during a separate hospitalization. For each positive blood culture, a chart review was performed to determine if the patient had received an ophthalmic examination while hospitalized. For patients having received an eye exam, we recorded visual acuity, fundus findings, presence of ocular symptoms, and fungal species. Of note, not all patients were able to relay symptoms or have visual acuity assessed due to level of consciousness. Patients who were first seen in the outpatient eye clinic and were subsequently admitted to the hospital were also included in the study. A diagnosis of chorioretinitis was made when deep focal white infiltrates were seen in the choroid or retina. The finding of vitreous extension of chorioretinal lesions, vitritis, or the presence of a vitreous abscess known as “fluff balls,” supported the diagnosis of endophthalmitis. Non-specific findings such as intraretinal haemorrhages, Roth spots, cotton-wool spots, and exudates, without findings of chorioretinitis or endophthalmitis, underwent serial dilated fundus exams in frequencies determined by the vitreoretinal specialists. An ophthalmology resident performed the initial examinations, and a vitreoretinal specialist subsequently confirmed the diagnoses. Patients younger than 18 years old were excluded from the study.
Results
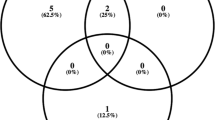
We identified 324 positive fungal cultures in 290 patients. Of this initial group, there were 161 eye exams involving 143 patients. Microbiology studies showed that positive cultures consisted of 153 Candida species and 9 non-Candida species. The most common Candida species were C. albicans (52) followed by C. glabrata (44). Ocular examination identified 7 of 161 patients (4.3%) with chorioretinitis or endophthalmitis. Three of four patients with endophthalmitis grew C. albicans. Two of four patients (50%) with chorioretinitis were either asymptomatic or unable to communicate symptoms. One patient had chorioretinitis in one eye and endophthalmitis in the fellow eye. A second patient had bilateral endophthalmitis. Three of four patients with endophthalmitis were seen in the outpatient eye clinic and diagnosed with endophthalmitis. All patients were then hospitalized for work-up and intravenous therapy, and later found to have candidemia. Once admitted to the hospital, two of these patients were subsequently found to have bacteremia, and one patient was found to have renal insufficiency. Only 42 (26%) patients had a 2-week follow-up exam; the follow-up exam did not reveal changes in any of the patients. For patients with positive exams that were able to participate in visual acuity testing, visual acuity did not decline or change significantly on follow-up visits after initiation of antifungal treatment. Two patients who developed endophthalmitis were not screened within the initial 7-day window recommended by IDSA. One of these patients was an inpatient but received fundus examination after the 1-week period and only after ocular symptoms developed. The other patient was discharged from the hospital without an eye exam and presented to the outpatient eye clinic with bilateral endophthalmitis.
Discussion
Overall incidence of fungal chorioretinitis and endophthalmitis was low in our study population, with an incidence of 4.3%. If we include only patients that were seen for screening eye exams based on a positive blood culture and exclude the three patients seen for vision loss in the outpatient eye clinic prior to hospitalization, incidence of positive eye findings in hospitalized patients would have decreased to 2.5% (4 of 158 inpatients). In this study, ocular involvement was identified in one out of every forty patients screened. Although comprising a small number, it should be noted that two patients who were incompletely screened went on to develop endophthalmitis. The majority of positive fungal cultures in the study group grew Candida species. There is less evidence of hematogenous intraocular spread for non-Candida species, and no official guidelines exist regarding necessity of fundus exams on patients with non-Candida fungal bloodstream infections. There were only nine patients in the study with a non-Candida species infection; a larger study population is needed to report the incidence and examine whether fundus exams are needed for this group.
Ability to verbalize ocular symptoms has been shown to have low sensitivity for screening patients for ocular candidiasis. Of our patients with ocular involvement, one patient was asymptomatic, and another was intubated and therefore unable to communicate. Studies that defined ocular involvement similar to our study have shown that a large number of the patients with ocular involvement are either asymptomatic or unable to communicate. Dozier et al. had a low incidence of ocular involvement (2/211, 0.9%), yet one of the patients was unable to communicate [10]. Adam et al. had an incidence of 11/227 (4.8%) and two patients were asymptomatic and four patients were unable to communicate [1]. The reality that more than half of patients with ocular involvement in these studies were either asymptomatic or noncommunicative supports the need for screening.
Additional studies are required to identify the most common risk factors for development of intraocular infection. The question also arises as to whether all patients with candidemia should undergo ophthalmic examination or if efforts can be focused on the group of patients deemed most susceptible to hematogenous spread.
Our study supports the guidelines set forth by both the IDSA and the AAO regarding ophthalmologic screening of patients with candidemia. The 4.3% combined incidence of chorioretinitis and endophthalmitis described in our study population is comparable to the 0.9–12.5% demonstrated in other studies [1, 3,4,5,6,7]. Significant to our dataset was the inclusion of two patients who were not screened appropriately and went on to develop endophthalmitis. These outcomes along with previous studies demonstrating a high rate of chorioretinitis and endophthalmitis in asymptomatic or noncommunicative patients reinforces the need for screening dilated fundus exams for patients with bloodstream Candida infections.
References
Adam MK, Sina V, Nichols M, Fintelmann R, Keenan J, Garg S, et al. Inpatient ophthalmology consultation for fungemia: prevalence of ocular involvement and necessity of funduscopic screening. Am J Ophthalmol. 2015;160.5:1078–83.
Brooks RG. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch Intern Med. 1989;149:2226–8.
Donahue S, Greven C, Zuravleff J, Eller A, Nguyen H, Peacock J, et al. Intraocular candidiasis in patients with candidemia. Ophthalmology. 1994;101:1302–9.
Feman S, Nichols J, Chung S, Theobald T. Endophthalmitis in patients with disseminated fungal disease. Trans Am Ophthalmological Soc. 2002;100:67–71.
Henderson DK, Edwards JE, Montgomerie JZ. Hematogenous Candida endophthalmitis in patients receiving parenteral hyperalimentation fluids. J Infect Dis. 1981;143:655–61.
Khalid A, Clough L, Symons RC, Mahnken J, Dong L, Eid A. Incidence and clinical predictors of ocular candidiasis in patients with Candida fungemia. Interdiscip Perspect Infect Dis. 2014;2014:1–6.
Karmisholt M, Hjort U, Knudsen L, Schønheyder H. Candidaemia and risk of intraocular infection: a Danish Hospital-Based Cohort Study. Scand J Infect Dis. 2008;40:241–6.
Huynh N, Chang HP, Borboli-Gerogiannis S. Ocular involvement in hospitalized patients with candidemia: analysis at a Boston Tertiary Care Center. Ocul Immunol Inflamm. 2012;20.2:100–3.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Zeichner-Ostrosky L, et al. Clinical Practice Guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2015;62:e1–50.
Dozier CC, Tarantola RM, Jiramongkolchai K, Donahue S. Fungal eye disease at a Tertiary Care Center: the utility of routine inpatient consultation. Ophthalmology. 2011;118:1671–6.
Ghodasra DH, Eftekhari K, Shah A, VanderBeek B. Outcomes, impact on management, and costs of fungal eye disease consults in a tertiary care setting. Ophthalmology. 2014;121:2334–9.
Krishna R, Amuh D, Lowder CY, Adal KA, Hall G. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye. 2000;14:30–4.
Vinikoor MJ, Zoghby J, Cohen K, Tucker J. Do all candidemic patients need an ophthalmic examination? Int J Infect Dis. 2013;17:e146–8.
Acknowledgements
We acknowledge Jones Eye Institute, University of Arkansas for Medical Sciences College of Medicine.
Funding
This work was supported by Research to Prevent Blindness and the Martha Wood Bentley Chair in Ophthalmology.
Author information
Authors and Affiliations
Contributions
MZS and GMG analysed data and wrote and edited portions of the manuscript. KTA wrote and edited portions of the manuscript. SHU and ABS were the attending physicians for all patients and wrote and edited the manuscript. ERR assisted with data collection and editing manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddiqui, M.Z., Gebhard, G.M., Ahmad, K.T. et al. Incidence of chorioretinitis and endophthalmitis in hospitalized patients with fungemia. Eye 36, 206–208 (2022). https://doi.org/10.1038/s41433-021-01477-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01477-2