Abstract
Purpose
To compare outcomes 1 year after accelerated cross-linking (CXL) between keratoconus eyes with central cones to those with paracentral cones.
Methods
In this post hoc analysis of data from a prospective multicentre study, consecutive progressive keratoconus eyes treated with accelerated CXL were included. Preoperative and 1 year post CXL manifest refraction, corneal cylinder, maximal keratometry (Kmax), central corneal thickness and coma were assessed. Central and paracentral cones were defined as cones within the central 3 mm and those between 3 and 5 mm, respectively. Eyes with apical scarring and peripheral cones (>5 mm) were excluded. The primary outcome measures were changes in best spectacle-corrected visual acuity (BSCVA) and Kmax.
Results
Overall, 314 eyes (n = 314) with a mean age of 27.5 ± 7.7 years were included. At baseline, the central cone group was younger (p < 0.001), had lower corneal astigmatism (p = 0.03) and coma (p = 0.02). At 1 year post CXL, after adjusting for baseline characteristics (age, BSCVA, corneal astigmatism, Kmax and coma), the central cone group showed a greater reduction in myopia (mean difference 1.27 ± 0.60D, p = 0.04) and more improvement in BSCVA (mean difference 0.08 ± 0.02 logMAR, p < 0.001) compared to the paracentral group. There was no significant difference in progression rates between the central and paracentral groups (ΔKmax > 2D, 6.7% vs. 6.5%, respectively, p = 0.83).
Conclusions
This large-scale study of keratoconus eyes 1 year after accelerated CXL indicates that compared to those with paracentral cones, central cones have on average almost one additional line improvement in BCSVA and 1.27 D more reduction in myopia.
Similar content being viewed by others
Introduction
Keratoconus is a non-inflammatory corneal ectasia of the central or paracentral region leading to corneal thinning, steepening and scarring [1]. In 2003, Wollensak et al. first introduced corneal collagen cross-linking (CXL) using riboflavin and ultraviolet A (UVA) as a new potential treatment for progressive keratoconus [2]. Several reports have demonstrated the efficacy of CXL in halting or delaying keratoconus progression by increasing corneal biomechanical stability [3,4,5,6,7].
Keratoconus is defined as central when the steepest point of the cornea is located in the central 3 mm zone, and paracentral when the steepest point is located outside of the central 3 mm zone. There have been conflicting reports as to whether cone location is a factor effecting CXL outcomes in keratoconus patients [5, 8,9,10,11]. Greenstein et al. reported that cone location was not associated with postoperative maximum keratometry (Kmax) [8]. Similarly, Toprak et al. reported that cone location had no effect on change in Kmax [5]. These were followed by a prospective study performed by Wisse et al. who reported an association between preoperative cone eccentricity and Kmax outcomes following CXL [9]. Afterwards, in a larger retrospective study, Koc et al. refuted such an association [10], while Sarac et al. reported an association [11].
To the best of our knowledge, no previous study has assessed the role of cone location in outcomes of CXL using an accelerated protocol using prospectively collected data. The purpose of the current study is to compare outcomes of accelerated CXL between keratoconus eyes with central cones to those with paracentral cones in this large cohort.
Methods
Study participants
The data for this post hoc analysis were extracted from a study that was approved by the University Health Network, Sunnybrook Health Sciences Centre, and the University of Toronto Research Ethics Boards, and written informed consent had been obtained from all participants [12].
This post hoc analysis included consecutive participants aged 14 and above, that underwent CXL for the treatment of progressive keratoconus between June 2013 and March 2015, and that had follow-up data available at 1 year. Excluded were patients with apical scarring, previous corneal surgery, peripheral marginal degeneration and clinically significant ocular comorbidities, such as history of chemical injury or delayed epithelial healing, any retinal or optic nerve disease that could potentially affect visual acuity [12].
Data collection
The following data were included in the analysis: logMAR best spectacle-corrected visual acuity (BCSVA), Scheimpflug Corneal tomography (Pentacam, Oculus Instruments, Wetzler, Germany) and ultrasonic pachymetry (PacPen Pachymeter 24-5, Accutome, Malvern, USA). The following preoperative data were collected for both eyes: age, gender, history of ocular allergies, BSCVA, Kmax, manifest refractive astigmatism, corneal cylinder, central corneal thickness (CCT), minimal corneal thickness (MCT), spherical equivalent (SEQ) and coma aberration (Nidek OPD, Nidek Co., Japan). The postoperative 1-year data included: BSCVA, Kmax, manifest refractive astigmatism, corneal cylinder, CCT, MCT, SEQ and coma.
Central and paracentral cones
Central cones were defined as cones that were located within the central 3 mm. Paracentral cones were defined as cones within the 3–5 mm region. Peripheral cones (n = 2) were defined as cones >5 mm and were excluded from analyses as no meaningful analyses could be performed for this group of eyes and there was a concern that they may in fact represent pellucid marginal degeneration (PMD). For subjects in which both eyes had CXL, only one eye was randomly selected [13]. Corneal tomographies of eyes with paracentral cones were reviewed by two independent clinicians (MM and TT) to confirm that these were indeed cases of inferior keratoconus and not PMD. Corneal tomographies with a crab claw pattern in the sagittal curvature maps and typical band-like inferior corneal thinning and protrusion above the thinning were categorized as suspicious for PMD and excluded [14, 15].
Definitions of progression
In order to assess progression following CXL, progression after CXL was defined as an absolute increase in Kmax >2 D at 1 year.
Cross-linking technique
A standardized alcohol-assisted epithelium off accelerated protocol (9 mW/cm2 UVA, 365 nm, 10 min, cumulative dose of 5.4 J/cm2) was performed in all patients as previously described by our group (Table 1) [12].
Sample size calculation
Assuming an effect size of 0.50, an alpha error of 0.05, a power of 0.80 and an allocation ratio of 0.20 (between paracentral and central cones) a required minimum sample size of 38 and 190 was calculated for the paracentral and central cones, respectively.
Statistical analysis
Data were analyzed with the Minitab Software, version 17 (Minitab Inc, State College, PA). For comparison of continuous and categorical data the Student’s t test and chi-square test were used, respectively. To compare 1-year outcomes, for continuous data and categorical data, general linear model and binary logistic regression were used respectively to account for differences in baseline between the central and paracentral groups. For comparison of preoperative and postoperative continuous data within groups the paired t test was used. A two-sided p value of < 0.05 was considered as statistically significant.
Results
Overall, 314 eyes of 314 subjects with a mean age of 27.5 ± 7.7 years (range, 14.2–61.0) of which 70.4% (n = 221) were male were included in the analyses. Central cones were identified in 85.4% (n = 268) of the eyes and the remainder (n = 47) were paracentral. One of the paracentral eyes was excluded from all analysis after being categorized by both reviewers as suspicious for PMD, whereas the rest (n = 46) were classified as paracentral keratoconus (100% agreement between reviewers).
Central and paracentral group baseline characteristics
Table 2 depicts a comparison of baseline characteristics between the central and paracentral cone groups. Briefly, the central group was younger (26.6 ± 7.1 years vs. 32.4 ± 9.2 years, p < 0.001), had less corneal astigmatism (3.3 ± 2.0 D vs. 4.0 ± 1.9 D, p = 0.03) and coma (2.88 ± 1.75 vs. 3.63 ± 1.85, p = 0.02). There were no significant differences in terms of seasonal or environmental allergies (38.1% vs. 37.0%, p = 0.89) or keratoconus grading (p = 0.98).
Comparison of 1 year outcomes between groups
Table 3 depicts a comparison of outcomes at 1 year between the central and paracentral cone groups before and after adjusting for baseline differences between groups (age, BSCVA, corneal astigmatism, Kmax and coma). The central group had a lower mean spherical equivalent (−2.84 ± 3.75 D) compared to the paracentral group (−3.66 ± 4.88D) (p = 0.28), for a mean difference of 1.27 ± 0.60 D (p = 0.04) after adjusting for baseline differences. The central group also had a better mean BSCVA (0.21 ± 0.19 logMAR) compared to the paracentral group (0.31 ± 0.25 logMAR) (p = 0.02), for a mean difference of 0.08 ± 0.02 logMAR (p < 0.001) after adjusting for baseline differences. There was no significant difference in Kmax progression (>2 D) rates at 1 year (6.7% vs. 6.5%, p = 0.83, adjusted p = 0.83).
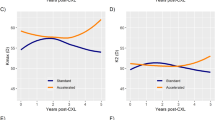
Change in outcomes at 1 year
Table 4 depicts change in outcomes at 1 year between the central and paracentral cone groups. There was a significant reduction in spherical equivalent only in the central group (0.68 ± 2.93, p < 0.001) and this reduction was significantly greater than the paracentral group (p = 0.05). In addition, the central group only had an improvement in BSCVA (−0.05 ± 0.16 logMAR, p < 0.001) and this was also significantly larger than the paracentral group (p = 0.04). Both groups remained stable at 1 year in terms of Kmax (p = 0.66 and p = 0.99, respectively).
Discussion
This large-scale study compared outcomes of CXL in keratoconus eyes with central versus paracentral cones. Subjects with central cones were younger, had lower corneal astigmatism and lower coma values. There was a modest improvement (less than one line) in BSCVA, as well as a reduction in myopia in the central cone group only. There were no significant differences between the central and paracentral cone groups with respect to progression rates. This is to the best of our knowledge the largest study to compare central versus paracentral cones in keratoconic eyes after CXL and the first prospective study to do so with an accelerated CXL protocol (Table 5).
The location of the steepest point of the cone in keratoconus was first reported by Ertan et al. [16]. They reported that younger patients had a vertical bowtie pattern, while the middle and older aged patients had an inferior global cone or inferotemporal global cone pattern, respectively [16]. Indeed, in the current study the paracentral cone group was significantly older than the central cone group. Considering that non-central keratoconus, particularly in older patients, may in fact represent overlooked PMD, all cases originally categorized by the investigators as keratoconus with a paracentral cone were reviewed to confirm they were not in fact PMD. All but one were confirmed to be paracentral keratoconus and not PMD.
In the current study, following CXL, there was a significant improvement in BSCVA in the central cone group only. After controlling for baseline differences, the central cone group had a four-letter improvement (0.08 logMAR) compared to the paracentral cone group. This is supported by the findings of Greenstein et al. who reported the greatest improvement in BSCVA in central cones following CXL [17]. Although they reported a significant improvement in both central and paracentral cones, the magnitude of the difference between both groups was somewhat similar to that of our study (0.06 logMAR) [17]. Similarly, Toprak et al. reported a significant improvement in BSCVA in the central group only (0.13 logMAR) whereas there was no significant improvement in the paracentral group (0.03 logMAR) [5]. In a multivariate analyses that assessed the correlation between cone eccentricity and change in BSCVA, no significant correlation was found between the two [9, 10]. It can be speculated that the minor difference in improvement in BSCVA between central and paracentral cones is likely due to the involvement of the visual axis and likely to be statistically significant in studies with larger numbers.
Discrepancies in findings have been reported regarding whether cone location is a predictor of change in Kmax following CXL. In the current study, there were no significant differences in baseline, 1 year and change in Kmax between the central and paracentral cone groups. In addition, there was no significant difference in Kmax progression rates at 1 year between groups. There was however a significant reduction in SEQ in the central cone group only, with the central cone group demonstrating 1.27 D greater reduction in SEQ. Greenstein et al. initially reported more topographic flattening in central cones [17], and later reported that when accounting for preoperative Kmax, the cone location no longer remained a significant predictor [8]. Several other studies reported superior Kmax outcomes in central cones compared to peripheral cones following CXL [5, 9, 11] with Koc et al. reporting that baseline Kmax was a more significant predictor with a greater effect [10]. The findings of the current study indicate that keratoconic eyes with central cones have a greater overall flattening of the cornea and a reduction in myopia but not necessarily a greater reduction in Kmax.
Two previous prospective studies have addressed cone location and its potential effects on CXL (Table 4) [8, 9]. Both of these studies reported outcomes of a 30 min Dresden protocol whereas the current study reports outcomes of an accelerated 10 min protocol, one that has gained popularity over recent years and has been shown to have similar outcomes to the original Dresden protocol [18]. In addition, the two previous studies consisted of smaller sample sizes [8, 9] with the study from Greenstein et al. including postoperative ectasia patients as well as keratoconus patients, perhaps making it difficult to apply separate conclusions to each population [8]. Furthermore, both studies included, for some patients, both eyes in the analysis and although the appropriate adjustments were made to account for this, these often come at the expense of statistical power [19]. In the current study, only patients with keratoconus were included and only one eye was included for each patient. Wisse et al. (n = 102) reported that only preoperative visual acuity was a predictor of postoperative visual acuity and that cone eccentricity was not a predictor in regression analysis [9]. In the current study, the large sample size (n = 314) enabled us to investigate more factors; the rule of thumb being that there should be a minimum of 10 observations per variable entering a multivariate model to avoid overfitting and leading to results that are not generalizable [20]. Indeed, in the current study, which was more sufficiently powered, cone location was a predictor of postoperative visual acuity.
This study has several limitations. First, the findings of this study directly apply only to accelerated epithelium off CXL, however these findings can likely be extrapolated to other protocols, such as standard epithelium off CXL and epithelium on CXL [21,22,23]. Second, habitual eye rubbing [24, 25] and floppy eyelid syndrome were not captured and may have played a role in the differences in outcomes between groups and it was not possible to quantify or qualify its effects. We did however adjust for seasonal and environmental allergies as a proxy measure of eye rubbing. Third, the two groups were uneven in size with 268 central cones and 46 paracentral cones, this is however considered a normal distribution of cone location for keratoconus patients [26]. Last, there were significant differences between the two groups at baseline and although statistical methods were used to adjust for these differences, they were not likely eliminated.
In summary, this large-scale study of keratoconus eyes 1 year after accelerated CXL indicates that compared to those with paracentral cones, central cones have on average almost one additional line improvement in BCSVA and 1.27 D more reduction in myopia. We feel that this paper will help surgeons use this information to counsel their patients regarding predicted outcomes of accelerated CXL.
Summary
What was known before
-
Keratoconus is a non-inflammatory corneal ectasia of the central or paracentral region leading to corneal thinning, steepening and scarring.
-
There have been conflicting reports as to whether cone location is a factor effecting CXL outcomes in keratoconus patients.
What this study adds
-
First study to compare outcomes of central and paracentral cones with an accelerated CXL protocol.
-
Central cones have on average almost one additional line improvement in BCSVA and 1.27 D more reduction in myopia.
References
Mathew JH, Goosey JD, Bergmanson JPG. Quantified histopathology of the keratoconic cornea. Optom Vis Sci. 2011;88:988–97.
Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7.
Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801.
Brooks NO, Greenstein S, Fry K, Hersh PS. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38:615–9.
Toprak I, Yaylali V, Yildirim C. Factors affecting outcomes of corneal collagen crosslinking treatment. Eye. 2014;28:41–46.
Lamy R, Netto CF, Reis RG, Procopio B, Porco TC, Stewart JM, et al. Effects of corneal cross-linking on contrast sensitivity, visual acuity, and corneal topography in patients with keratoconus. Cornea. 2013;32:591–6.
Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: The Siena Eye Cross Study. Am J Ophthalmol. 2010;149:585–93.
Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: Implications for patient selection. J Cataract Refract Surg. 2013;39:1133–40.
Wisse RPL, Godefrooij DA, Soeters N, Imhof SM, Van, der Lelij A. A multivariate analysis and statistical model for predicting visual acuity and keratometry one year after cross-linking for keratoconus. Am J Ophthalmol. 2014;157:519–.e2.
Koc M, Uzel MM, Tekin K, Kosekahya P, Ozulken K, Yilmazbas P. Effect of preoperative factors on visual acuity, corneal flattening, and corneal haze after accelerated corneal crosslinking. J Cataract Refract Surg. 2016;42:1483–9.
Sarac O, Caglayan M, Cakmak HB, Cagil N. Factors influencing progression of keratoconus 2 years after corneal collagen cross-linking in pediatric patients. Cornea. 2016;35:1503–7.
Hatch W, El-Defrawy S, Ong Tone S, Stein R, Slomovic AR, Rootman DS, et al. Accelerated corneal cross-linking: efficacy, risk of progression, and characteristics affecting outcomes. a large, single-center prospective study. Am J Ophthalmol. 2020;213:76–87.
Holopigian K, Bach M. A primer on common statistical errors in clinical ophthalmology. Doc Ophthalmol. 2010;121:215–22.
Koc M, Tekin K, Inanc M, Kosekahya P, Yilmazbas P. Crab claw pattern on corneal topography: Pellucid marginal degeneration or inferior keratoconus? Eye 2018;32:11–8.
Martínez-Abad A, Piñero DP. Pellucid marginal degeneration: detection, discrimination from other corneal ectatic disorders and progression. Contact Lens Anterior Eye. 2019;42:341–9.
Ertan A, Kamburoglu G, Colin J. Location of steepest corneal area of cone in keratoconus stratified by age using Pentacam. J Refract Surg 2009;25:1012–6.
Greenstein SA, Fry KL, Hersh PS. Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg. 2012;28:397–405.
Kymionis GD, Kontadakis GA, Hashemi KK. Accelerated versus conventional corneal crosslinking for refractive instability: an update. Curr Opin Ophthalmol. 2017;28:343–7.
Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33:7–14.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstem AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9.
Lang PZ, Hafezi NL, Khandelwal SS, Torres-Netto EA, Hafezi F, Randleman JB. Comparative functional outcomes after corneal crosslinking using standard, accelerated, and accelerated with higher total fluence protocols. Cornea. 2019;38:433–41.
Hersh PS, Lai MJ, Gelles JD, Lesniak SP. Transepithelial corneal crosslinking for keratoconus. J. Cataract Refract. Surg. 2018;44:313–22.
Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal crosslinking without epithelial removal. J Cataract Refract Surg. 2018;44:1363–70.
Jafri B, Lichter H, Stulting RD. Asymmetric keratoconus attributed to eye rubbing. Cornea. 2004;23:560–4.
Hafezi F, Hafezi NL, Pajic B, Gilardoni F, Randleman JB, Gomes JAP, et al. Assessment of the mechanical forces applied during eye rubbing. BMC Ophthalmol. 2020;20:301.
Tian M, Ma P, Zhou W, Feng J, Mu G. Outcomes of corneal crosslinking for central and paracentral keratoconus. Medicine. 2017;96:e6247.
KEI CXL Study Group
Raymond Stein1, Matthew C. Bujak1, Clara C. Chan1, Hall F. Chew1, Sherif El-Defrawy1, Christoph Kranemann1, Theodore Rabinovitch1, David S. Rootman1, Allan R. Slomovic1.
Funding
The data from this study were part of a province—wide initiative funded by the Ontario Ministry of Health and Long-term Care to report on the safety and efficacy of CXL.
Author information
Authors and Affiliations
Consortia
Contributions
All authors were responsible for the conception of the work. All authors aided in acquisition, analysis and interpretation of data. MM drafted the work and all other authors critically revised it. All authors provided approval of the final version and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
MM: Lapidot Medical and EyeYon Medical. CCC: Alcon Laboratories Inc, Allergan, Bausch & Lomb, Santen, Shire, Tearlab, Labtician Thea and Johnson & Johnson. ARS: Abbott, Abbvie, Alcon Laboratories Inc, Allergan, Bausch & Lomb, Santen Inc, Shire, and Thea/Labtician. DSR: Alcon Laboratories Inc and Johnson & Johnson. TR: Alcon Laboratories Inc., Johnson & Johnson and Abbvie.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the KEI CXL Study Group are listed before Funding
Rights and permissions
About this article
Cite this article
Mimouni, M., Sorkin, N., Trinh, T. et al. Central versus paracentral cone location and outcomes of accelerated cross-linking in keratoconus patients. Eye 35, 3311–3317 (2021). https://doi.org/10.1038/s41433-021-01404-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01404-5