Abstract
The introduction of optical coherence tomography angiography (OCTA) has remarkably expanded our knowledge of the ocular vascular alterations occurring in diabetes. In this article, a review of the prominent OCTA findings in diabetes is followed by a description of salient histological and anatomical features of microaneurysms, essential for the proper interpretation of in vivo imaging of these retinal vascular abnormalities. The recent employment of a three-dimensional (3D) visualization in OCTA imaging is also discussed. The latter imaging technique has granted a detailed characterization of microaneurysms in vivo.
摘要
光学相干断层扫描血管成像(optical coherence tomography angiography, OCTA) 在临床的广泛应用加深了我们对糖尿病眼部血管改变的认识。本文首先对OCTA针对糖尿病的重要发现进行了回顾, 随后描述了微血管瘤显著的组织学及解剖学特征, 这对合理解释视网膜血管异常的体内成像至关重要。本文进一步讨论了OCTA中应用三维 (three-dimensional, 3D) 可视化成像的最新进展, 这种新的影像成像技术可实现描述活体微血管瘤的细节特征。
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a microangiopathy of the retinal vessels resulting from the chronic effects of diabetes. Although diabetes has several effects on the retinal tissue, including a direct effect on the neurons and support cells of the retina [1, 2], the retinal vascular abnormalities represent the main clinical manifestation. Diabetic retinopathy may be classified into non-proliferative (NPDR) and proliferative (PDR) stages.
Optical coherence tomography angiography (OCTA) is a noninvasive imaging technique that is able to quickly obtain angiographic images of the retinal and choroidal vasculature [3,4,5,6]. In this review, we will discuss recent applications of this imaging modality to diabetes. Of note, we will provide a more detailed description of the OCTA employment to characterize diabetic microaneurysms.
OCTA findings in diabetes
Retinal vasculature
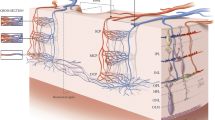
OCTA has significantly improved our capability to investigate and quantify the retinal perfusion. The retinal vascularization has a peculiar organization that may be divided into four plexuses: the radial peripapillary capillary plexus (RPCP), the superficial capillary plexus (SCP), the middle (or intermediate) capillary plexus (MCP), and the deep capillary plexus (DCP) [7,8,9,10]. The MCP and DCP are frequently grouped together into the deep vascular complex (DVC). The three latter plexuses (SCP, MCP, and DCP) delimitate the foveal avascular zone (FAZ) that is round-shaped or oval-shaped in healthy eyes.
In diabetic patients, the FAZ area was displayed to be larger, as compared with healthy subjects. Importantly, Vujosevic et al. [11] proved that also subjects with type 1 diabetes and without signs of DR may have an increase in FAZ size, the latter aspect highlighting the fact that this alteration is early in diabetic patients. Similarly, De Carlo and collegues [12] proved that also patients with type 2 diabetes and no signs of DR have a larger FAZ compared with normal subjects.
OCTA metrics have been widely used to demonstrate a reduced retinal perfusion in eyes with DR. Notably, OCTA analysis proved that diabetic patients without any signs of DR have a lower DCP perfusion density in comparison with controls [13]. In patients affected by type 2 diabetes and DR, OCTA metrics (perfusion and vessel length densities) were proved to be strictly associated with DR stage and visual acuity [14].
Moreover, OCTA metrics may be employed to monitor the retinal vascular impairment in diabetic patients by performing sequential scans throughout following visits. Repeated OCTA scans have been recently used by Alagorie et al. [15] to evaluate the association of intravitreal aflibercept with changes in macular perfusion in patients with PDR without diabetic macular oedema. The latter study demonstrated that macular perfusion is stable over 12 months of follow-up during treatment with intravitreal aflibercept. Assuming that nonperfusion is expected to progress in eyes with DR, the latter findings were speculated to represent a beneficial effect of anti–vascular endothelial growth factor (VEGF) therapy on retinal perfusion, as also suggested using fluorescein angiography (FA) [16].
Also, OCTA metrics have been recently demonstrated to be beneficial in predicting DR disease progression. In a prospective study on 73 subjects, the authors detected a disease progression in 15 out of 73 patients over a follow-up period of 12 months [17]. Importantly, the latter study demonstrated that a larger FAZ area, presence of intraretinal abnormalities (IRMA), and reduced peripapillary perfusion at baseline were significantly associated with increased odds of progression.
Although OCTA may provide a quantification of retinal perfusion, this imaging modality has also been employed to describe and characterize DR-associated vascular abnormalities, including venous loops, intraretinal microvascular abnormalities, and retinal neovascularizations. In details, venous loops are detected as lesions with flow in proximity to regions of hypoperfusion, in agreement with the ischemic pathogenesis of these lesions [18]. Moreover, OCTA is able to visualize IRMAs as tiny retinal vascular networks with focal areas of increased blood flow within the SCP and in proximity to ischemic regions [19, 20]. OCTA is also useful in distinguishing retinal neovascularizations from IRMAs by examining OCTA B-scans [21]. Using OCTA, Shimouchi and colleagues [22] identified five subtypes of IRMAs (unchanged, tuft regression, reperfusion, mixed [combined tuft regression/reperfusion], and worsening with new appearance of tuft) on the basis of IRMAs’ modifications after panretinal photocoagulation (PRP). Noteworthy, the authors demonstrated that some IRMAs did not exhibit morphological changes before and after PRP, this finding suggesting that some IRMAs might represent vascular remodeling of existing capillaries rather than intraretinal neovascularizations [22]. OCTA imaging also grants the visualization of retinal and optic disc neovascularization that are displayed as well-delineated microvascular structures of new vessels [23]. Importantly, on OCTA images, most new vessels were visualized as irregular proliferation of fine vessels, which were defined as exuberant vascular proliferation. Presence of exuberant vascular proliferation was suggested as an OCTA sign of neovascular leakage.
Widefield OCTA
Many OCTA devices have been implemented with the capability to capture widefield images of the retinal and choroidal vasculature (Fig. 1). Assuming that widefield FA imaging modality has been historically considered as the gold-standard in detecting DR-associated retinal neovascularizations, several studies have compared widefield OCTA images with widefield FA images in order to validate the former modality in DR patients [24,25,26]. Overall, these studies demonstrated that widefield OCTA can detect small neovascularizations not seen on clinical examination or color photographs and may therefore improve the clinical assessment of patients with DR. Importantly, rates of detection of neovascularizations using either widefield OCTA or FA were comparable [25]. However, the widefield OCTA evaluation is still limited by artifacts [27, 28] and in the inability of OCTA in assessing neovascular leakage and in covering the whole retinal field, as compared with widefield FA (i.e., especially in comparison with ultra-widefield FA systems) [29]. This may result in an underestimation of the retinal ischemia. Widefield OCTA was also compared with ultra-widefield color fundus photography and widefield FA for detecting other DR-associated vascular lesions (i.e., microaneurysms, intraretinal microvascular abnormalities, and non-perfusion areas) [25]. The latter study’s results proved that detection rates of these lesions were high using widefield OCTA.
a Ultra-pseudocolor image demonstrated retinal hemorrhages, microaneurysms, cotton wool spots, hard exudates, and IRMA (intraretinal microvascular abnormalities). b, c In the ultra-widefield fluorescein angiography early-phase and late-phase images, numerous regions of hyperfluorescence with leakage may be seen corresponding to areas with microaneurysms and retinal neovascularization. Staining of pan-retinal laser photocoagulation scars are also evident. d The widefield en face OCTA image including the whole retina has vessels of different colors based on their depth. This image demonstrates regions of retinal ischemia (highlighted with blue arrowheads). e The OCTA image segmented at the vitreous level demonstrates the presence of a neovascularization at the level of the optic disc. Ultra-widefield pseudocolor and fluorescein angiography images were obtained with the California Optos device (Dunfermline, United Kingdom). The widefield OCTA images were performed with the Plex Elite 9000 device (Carl Zeiss Meditec Inc., Dublin, CA, USA).
Retinal perfusion using 3D OCTA
While OCTA data are mostly displayed with en face two-dimensional (2D) images, a three-dimensional (3D) analysis may allow a reliable visualization and quantification of the retinal perfusion [30,31,32,33,34]. Importantly, 2D images are obtained by flattening of data in any given specific space (e.g., SCP) [3,4,5]. However, this process may result in an undervaluation of flow as overlapping vessels segmented in the same space may erroneously be merged. An additional limitation of 2D images is that retinal vessels crossing different slabs may also be erroneously visualized twice on two different OCTA images (e.g., SCP and DVC) [32]. Finally, the en face 2D visualization may be significantly affected by segmentation artifacts, especially in pathological conditions [35]. The latter limitation was demonstrated to significantly influence the repeatability of 2D OCTA metrics in eyes with DR [36, 37].
Differently, a 3D visualization does not need flattening of flow information and it is independent of data segmentation [32, 33]. Our group has recently adopted a methodology to visualize the macular retinal vasculature in 3D and successively we obtained two novel 3D OCTA metrics: (i) 3D vascular volume; and (ii) 3D perfusion density [34]. In the latter study, 15 diabetic patients and 15 healthy subjects were retrospectively enrolled and their OCTA data were processed for generating 2D and 3D OCTA metrics. Results from the latter study illustrated that 2D and 3D parameters are significantly associated, and 3D quantitative metrics differ between patients and controls. Importantly, 3D OCTA metrics were characterized by elevated inter-observer agreement levels. Based on these findings and assuming that 2D en face OCTA images are limited by underestimation/overestimation of flow and segmentation errors, we concluded that the possibility to quantify diabetic macular ischemia using 3D analysis is promising.
The choriocapillaris
Several studies have proved that the CC may be significantly impaired in diabetic patients and that this damage may be partially involved in the increased vascular endothelial growth factors (VEGF) levels in these eyes [4, 38,39,40]. In details, the CC perfusion was demonstrated to be reduced in both NPDR and PDR eyes, as compared with healthy controls [39]. Noteworthy, similar changes were also displayed in a subgroup of patients with diabetes and no signs of DR [39]. Furthermore, the reduced CC perfusion was demonstrated to be significantly dependent on the DR stage, with advanced stages characterized by a more significant CC hypoperfusion [39]. Finally, two different papers using OCTA and structural OCT proved that the CC hypoperfusion in diabetic patients is significantly associated with photoreceptor damage in NPDR patients, this finding highlighting the importance of CC impairment in macular dysfunction in these eyes [40, 41].
Microaneurysms
Histopathologic findings
Microaneurysms represent capillaries’ dilations which usually emerge as gross outpouchings of the vessel wall. Their size varies from 25 to 100 μm in diameter [42]. Although these vascular malformations may be noticed in several vascular beds (e.g., kidney, heart), they have been historically assessed in the retinal vasculature of diabetic patients [43]. Importantly, retinal microaneurysms often represent the earliest ophthalmoscopic finding that may be visualized in eyes with DR [44].
The mechanisms driving microaneurysms’ formation are not well understood and include diabetes-associated modifications in the retinal microenvironment, impairment of the endothelial cells owing to leukostasis, loss of pericytes, and increase in intraluminal pressure [42].
Microaneurysms have been extensively characterized by histopathologic studies [42, 43, 45, 46]. Their lumen was demonstrated to be often occluded by agglutinated erythrocytes or thrombus [42]. Furthermore, microaneurysms may become acellular without endothelial cells and pericytes [42]. In 1999, Moore et al. [46] combined an immunohistochemical technique with confocal laser scanning microscopy to characterize microaneurysms without modifying the tissue architecture. The latter study proved that the majority of microaneurysms had two retinal vessels associated. Furthermore, this ex vivo analysis on diabetic patients displayed that the majority of microaneurysms arise in the inner nuclear layer (INL) and therefore they mostly originate in the deeper part of the retinal circulation [46]. Moreover, microaneurysms were revealed to be characterized by different shapes and three different morphologic patterns could be discerned: (i) saccular, (ii) fusiform, and (iii) focal bulges [46].
OCTA findings
OCTA analysis confirmed previous histopathological findings, as microaneurysms were demonstrated to be mainly included in the DVC. On 2D OCTA images, microaneurysms may appear as saccular or fusiform capillary dilations [47,48,49].
Using structural OCT and OCTA, our group has fully characterized diabetic microaneurysms [48, 49]. On structural OCT, these vascular lesions may have variable internal reflectivity as they may be characterized by an hyperreflective, intermediate reflective or hyporeflective content [48]. The internal reflectivity may modify throughout the follow-up [48]. Noteworthy, the structural OCT characteristics were demonstrated to influence the OCTA capability to detect microaneurysms, as lesions with an hyporeflective content are less prone to be detected on OCTA images [48]. The latter feature is probably dependent on the slow flow rate within these hyporeflective microaneurysms, which is lower than that detectable using OCTA [48]. Conversely, hyperreflective microaneurysms are more frequently visualized using OCTA, this probably reflecting an higher blood flow rate within these microaneurysms [48]. Assuming that a faster blood flow may cause a damage of the blood-retinal barrier with consequent fluid accumulation, in a successive paper we demonstrated that the presence at baseline of hyperreflective microaneurysms, which are more frequently seen on OCTA, is associated with a higher probability of extracellular fluid accumulation at 1 year of follow-up [49]. Finally, these two studies [48, 49] confirmed previous findings suggesting that microaneurysms may be also located in the outer retinal vasculature [50].
The visualization of microaneurysms on OCTA images is also strictly dependent on the device employed [51]. Parrulli et al. [51] performed a nice study comparing FA with five OCTA devices in detecting microaneurysms. The authors included 20 eyes of 15 patients with DR and demonstrated that, while fluorescein angiography remains the best imaging modality to detect retinal microaneurysms, OCTA devices have different rates of detection. In details, the Spectralis OCTA (Heidelberg Engineering, Heidelberg, Germany) was able to detect more microaneurysms compared to other devices. The authors speculated that this finding was secondary to the higher number of B-scans in the scanned area and to the higher number of repeated B-scans employed by the Spectralis device. Based on their findings, the authors concluded that the variability among OCTA devices to detect retinal microaneurysms should considered in clinical trials and in clinical practice [51]. Of note, multiple image averaging was demonstrated to be useful for increasing the microaneurysm detection OCTA capability [52].
Three-dimensional OCTA
As stated above, while OCTA data are mainly displayed using 2D cross-sectional or en face images, recent evidence suggests that a 3D analysis may permit a reliable visualization and quantification of the retinal vessels [30,31,32,33,34]. Importantly, a 2D OCTA visualization is limited in characterizing anatomical entities where both their en face extent and depth information is important to further comprehend their findings, such as microaneurysms in diabetes. These OCTA shortcomings have been addressed with the development of 3D illustrations [32].
Our group first applied a rotational 3D OCTA visualization to describe diabetic microaneurysms in vivo (Fig. 2) [32]. In the latter study, we retrospectively collected data from 20 patients (20 eyes) with diabetic retinopathy who had OCTA imaging obtained with the PLEX Elite 9000 device (Carl Zeiss Meditec Inc., Dublin, CA, USA). After processing the OCTA volume data with a novel volume projection removal algorithm, OCTA data were imported in imageJ software (National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) [53] in order to obtain a 3D visualization of the analyzed microaneurysms. Our analysis included fifty-two microaneurysms and, overall, this approach was demonstrated to be useful for an accurate characterization of these vascular malformations.
Our 3D analysis revealed that most microaneurysms occupy at least two retinal layers and the INL was revealed to be the retinal layer most frequently occupied by these vascular abnormalities [32]. Therefore, these results confirmed previous histopathological findings, as explained above [46]. Of note, these results were also in agreement with reports employing structural OCT [54, 55]. A previous study by Wang et al. [54] used structural OCT and nicely proved that most microaneurysms occupy more than one retinal layer. Successively, Karst et al. [55] added adaptive optics to OCT imaging in order to improve the transverse resolution and further confirmed these findings.
Given that a single microaneurysm may be included in different retinal layers, a 2D OCTA visualization may be prone to visualize the same microaneurysm on two distinct 2D en face OCTA images using different slabs (e.g., SCP and DVC). Therefore, a 2D OCTA visualization may result in the same microaneurysm counted twice, as demonstrated in a previous study [47].
Our study with 3D OCTA on microaneurysms also proved that most of analyzed microaneurysms are associated with two vessels [32], this finding suggesting no tendency to develop at vascular junctions, in agreement with histology [46].
One of the most relevant results of our 3D analysis was that, even though most microaneurysms are located in the INL, where the DVC is accommodated, a number of microaneurysms was graded to represent dilations of vessels arising from the SCP rather than the DVC [32]. Of note, a small number of microaneurysms was rated to be connected with both SCP and DVC vessels, this aspect likely reflecting the eventuality of microaneurysms to develop at the level of vessels connecting the SCP and DVC vascular beds [32].
Finally, the 3D analysis provided a way to investigate the spatial orientation of microaneurysms with respect to the retinal layers [32]. In detail, each microaneurysm was characterized by a peculiar orientation on the three dimensions and most microaneurysms had an oblique orientation as they had angles >0 with the three axes of the three-dimensional Cartesian coordinate system [32]. Considering that microaneurysms traverse different retinal layers, we speculated that this oblique orientation was secondary to the presence of Müller cells, whose processes are known to be featured by an oblique orientation [32]. Of note, the 3D visualization of microaneurysms demonstrated that those microaneurysms with a horizontal orientation were mainly included in the ganglion cell complex (GCC) or outer plexiform layer (OPL), this probably reflecting the presence of horizonal cells within these two layers [32].
Conclusions
OCTA has been significantly employed to better characterize vascular alterations in diabetes and remarkable insights have already been achieved with this technology.
Several studies have identified retinal and choroidal hypoperfusion in diabetic eyes. More importantly, quantitative analysis of the macular ischemia with OCTA may provide an important biomarker of disease. The employment of OCTA to detect areas of hypoperfusion of the retinal periphery cannot be underestimated, although widefield OCTA systems are still limited in the assessment of the periphery in comparison with ultra-widefield fluorescein angiography imaging modality. Finally, OCTA technology employing 3D analysis have already provided incredible in vivo visualizations of microaneurysms that, for the first time, resemble the histopathological representations.
In summary, OCTA represents a powerful tool to characterize and assess in the clinical practice patients with diabetic retinopathy.
References
De Benedetto U, Querques G, Lattanzio R, Borrelli E, Triolo G, Maestranzi G, et al. Macular dysfunction is common in both type 1 and type 2 diabetic patients without macular edema. Retina. 2014;34:2171–7.
Carpineto P, Toto L, Aloia R, Ciciarelli V, Borrelli E, Vitacolonna E, et al. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye. 2016;30:673–9.
Spaide RRF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2017;64:1–55.
Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30–55.
Borrelli E, Sadda SR, Uji A, Querques G. Pearls and pitfalls of optical coherence tomography angiography imaging: a review. Ophthalmol Ther. 2019;8:215–26.
Borrelli E, Sadda SR, Uji A, Querques G. OCT angiography: guidelines for analysis and interpretation. In: OCT and Imaging in Central Nervous System Diseases. Springer; 2020. p. 41–54
Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015;35:2196–203.
Garrity ST, Paques M, Gaudric A, Freund KB, Sarraf D. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina. 2017;1809–12.
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201.
Nesper PL, Fawzi AA. Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2018;59:3858–67.
Vujosevic S, Muraca A, Alkabes M, Villani E, Cavarzeran F, Rossetti L, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019;39:435–45.
De Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70.
Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54:695–702.
Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124:235–44.
Alagorie AR, Nittala MG, Velaga S, Zhou B, Rusakevich AM, Wykoff CC, et al. Association of intravitreal aflibercept with optical coherence tomography angiography vessel density in patients with proliferative diabetic retinopathy. JAMA Ophthalmol. 2020;138:851–7.
Borrelli E, Sacconi R, Querques G, Re Couturier, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti–vascular endothelial growth factor. Ophthalmology. 2019;126:1685–94.
Greig EC, Brigell M, Cao F, Levine ES, Peters K, Moult EM, et al. Macular and peripapillary OCTA metrics predict progression in diabetic retinopathy: a sub-analysis of TIME-2b Study Data. Am J Ophthalmol. 2020. https://doi.org/10.1016/j.ajo.2020.06.009.
Giuffrè C, Carnevali A, Cicinelli MV, Querques L, Querques G, Bandello F. Optical coherence tomography angiography of venous loops in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retin. 2017;48:518–20.
Elbendary AM, Abouelkheir HY. Bimodal imaging of proliferative diabetic retinopathy vascular features using swept source optical coherence tomography angiography. Int J Ophthalmol. 2018;11:1528.
Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160:35–44.
Lee CS, Lee AY, Sim DA, Keane PA, Mehta H, Zarranz-Ventura J, et al. Reevaluating the definition of intraretinal microvascular abnormalities and neovascularization elsewhere in diabetic retinopathy using optical coherence tomography and fluorescein angiography. Am J Ophthalmol. 2015;159:101–10.
Shimouchi A, Ishibazawa A, Ishiko S, Omae T, Ro-Mase T, Yanagi Y, et al. A proposed classification of intraretinal microvascular abnormalities in diabetic retinopathy following panretinal photocoagulation. Investig Ophthalmol Vis Sci. 2020;61:34.
Ishibazawa A, Nagaoka T, Yokota H, Takahashi A, Omae T, Song YS, et al. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57:6247–55.
Sawada O, Ichiyama Y, Obata S, Ito Y, Kakinoki M, Sawada T, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2018;256:1275–80.
Cui Y, Zhu Y, Wang JC, Lu Y, Zeng R, Katz R, et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br J Ophthalmol. 2020. https://doi.org/10.1136/bjophthalmol-2020-316245
You QS, Guo Y, Wang J, Wei X, Camino A, Zang P, et al. Detection of clinically unsuspected retinal neovascularization with wide-field optical coherence tomography angiography. Retina. 2020;40:891–7.
Borrelli E, Viggiano P, Evangelista F, Toto L, Mastropasqua R. Eyelashes artifact in ultra-widefield optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retin. 2019;50:740–3.
Cui Y, Zhu Y, Wang JC, Lu Y, Zeng R, Katz R, et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl Vis Sci Technol. 2019;8:18.
Battista M, Borrelli E, Sacconi R, Bandello F, Querques G. Optical coherence tomography angiography in diabetes: a review. Eur J Ophthalmol. 2020;30:411–6.
Spaide RF. Volume rendering of optical coherence tomography angiography reveals extensive retinal vascular contributions to neovascularization in ocular toxoplasmosis. Retina. 2015;35:2421–2.
Spaide RF, Suzuki M, Yannuzzi LA, Matet A, Behar-Cohen F. Volume-rendered angiographic and structural optical coherence tomography angiography of macular telangiectasia type 2. Retina. 2017;37:424–35.
Borrelli E, Sacconi R, Brambati M, Bandello F, Querques G. In vivo rotational three-dimensional OCTA analysis of microaneurysms in the human diabetic retina. Sci Rep. 2019;9:1–8.
Borrelli E, Sacconi R, Klose G, de Sisternes L, Bandello F, Querques G. Rotational three-dimensional OCTA: a notable new imaging tool to characterize type 3 macular neovascularization. Sci Rep. 2019;9:1–8.
Borrelli E, Sacconi R, Querques L, Battista M, Bandello F, Querques G. Quantification of diabetic macular ischemia using novel three-dimensional optical coherence tomography angiography metrics. J Biophoton. 2020:e202000152. https://doi.org/10.1002/jbio.202000152.
Ghasemi Falavarjani K, Habibi A, Anvari P, Ghasemizadeh S, Ashraf Khorasani M, Shenazandi H, et al. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br J Ophthalmol. 2020;104:162–6.
Czakó C, Sándor G, Ecsedy M, Récsán Z, Horváth H, Szepessy Z, et al. Intrasession and between-visit variability of retinal vessel density values measured with OCT angiography in diabetic patients. Sci Rep. 2018;8:1–8.
You Q, Freeman WR, Weinreb RN, Zangwill L, Manalastas PIC, Saunders LJ, et al. Reproducibility of vessel density measurement with optical coherence tomography angiography in eyes with and without retinopathy. Retina. 2017;37:1475.
Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–3.
Choi W, Waheed NK, Moult EM, Adhi M, Lee B, De Carlo T, et al. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina. 2017;37:11–21.
Borrelli E, Palmieri M, Viggiano P, Ferro G, Mastropasqua R. Photoreceptor damage in diabetic choroidopathy. Retina. 2019;40:1062–9.
Dodo Y, Suzuma K, Ishihara K, Yoshitake S, Fujimoto M, Yoshitake T, et al. Clinical relevance of reduced decorrelation signals in the diabetic inner choroid on optical coherence tomography angiography. Sci Rep. 2017;7:1–11.
Wiley HE, Ferris FL. Nonproliferative diabetic retinopathy and diabetic macular edema. 5th edn. In: Retina; 2012. Vol. 2, p. 940–68. https://doi.org/10.1016/B978-1-4557-0737-9.00047-3.
Ashton N. Studies of the retinal capillaries in relation to diabetic and other retinopathies. Br J Ophthalmol. 1963;47:521.
Feman SS. The natural history of the first clinically visible features of diabetic retinopathy. Trans Am Ophthalmol Soc. 1994;92:745.
Fryczkowski AW, Chambers RB, Craig EJ, Walker J, Davidorf FH. Scanning electron microscopic study of microaneurysms in the diabetic retina. Ann Ophthalmol. 1991;23:130–6.
Moore J, Bagley S, Ireland G, McLeod D, Boulton ME. Three dimensional analysis of microaneurysms in the human diabetic retina. J Anat. 1999;194:89–100.
Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Investig Ophthalmol Vis Sci. 2016:57:348–55.
Parravano M, De Geronimo D, Scarinci F, Querques L, Virgili G, Simonett JM, et al. Diabetic microaneurysms internal reflectivity on spectral-domain optical coherence tomography and optical coherence tomography angiography detection. Am J Ophthalmol 2017;179:90–6.
Parravano M, De Geronimo D, Scarinci F, Virgili G, Querques L, Varano M, et al. Progression of diabetic microaneurysms according to the internal reflectivity on structural optical coherence tomography and visibility on optical coherence tomography angiography. Am J Ophthalmol. 2019;198:8–16.
Querques G, Bandello F, Souied EH. Abnormal deep retinal capillary networking and microaneurysms in the outer nuclear layer of diabetic eyes. Ophthalmology. 2014;121:803–4.
Parrulli S, Corvi F, Cozzi M, Monteduro D, Zicarelli F, Staurenghi G. Microaneurysms visualisation using five different optical coherence tomography angiography devices compared to fluorescein angiography. Br J Ophthalmol. 2020. https://doi.org/10.1136/bjophthalmol-2020-316817.
Kaizu Y, Nakao S, Wada I, Arima M, Yamaguchi M, Ishikawa K, et al. Microaneurysm imaging using multiple en face OCT angiography image averaging: morphology and visualization. Ophthalmol Retin. 2020;4:175–86.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Wang H, Chhablani J, Freeman WR, Chan CK, Kozak I, Bartsch DU, et al. Characterization of diabetic microaneurysms by simultaneous fluorescein angiography and spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2012;153:861–7.
Karst SG, Salas M, Hafner J, Scholda C, Vogl W-D, Drexler W, et al. Three-dimensional analysis of retinal microaneurysms with adaptive optics optical coherence tomography. Retina. 2019;39:465–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Querques, G., Borrelli, E., Battista, M. et al. Optical coherence tomography angiography in diabetes: focus on microaneurysms. Eye 35, 142–148 (2021). https://doi.org/10.1038/s41433-020-01173-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01173-7
This article is cited by
-
Ophthalmic artery changes in type 2 diabetes with and without acute coronary syndrome
Journal of Translational Medicine (2022)
-
Ocular imaging at the cutting-edge
Eye (2021)