Abstract
Background/objective
To investigate the influence of pre-operative immunological and nutritional status, assessed by the prognostic nutritional index (PNI) score, on post-operative infection, and the potential additive effects of low PNI and infection on prognosis after radical resection of stage II/III gastric cancer (GC).
Methods
The medical records of 2352 consecutive stage II/III GC patients who underwent radical gastrectomy were retrospectively reviewed. The independent predictors for infections were identified using univariate and multivariate analyses. Cox regression analysis was used to assess any associations between PNI, infection and OS.
Results
A total of 160 (6.8%) cases developed infections and low PNI (< 43.9) was confirmed as an independent predictor. Both PNI < 43.9 and infections independently predicted poor OS (hazard ratio: 1.163, 95% confidence interval: 1.007–1.343; HR: 1.347, 95%CI: 1.067–1.700), and an additive effect was confirmed as patients with both low PNI and infection had worst OS. Further stratified analyses showed that complete peri-operative adjuvant chemotherapy (PAC, ≥ 6 cycles) could significantly improve OS in patients with low PNI and/or infection, which was comparable to those with PNI ≥ 43.9 and/or infection (P = 0.160).
Conclusions
Infection was the most common complication after gastrectomy and PNI < 43.9 was identified as an independent predictor. Low PNI was associated with poorer OS in stage II/III GC, independent of infections, and low PNI and infections had a synergistic effect that was associated with worst OS. However, complete PAC could significantly improve OS in these patients. Thus, strategies to decrease infection and complete PAC should be further investigated.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed malignancy and the fourth leading cause of tumor-induced death worldwide [1]. Unfortunately, more than half of GC patients are diagnosed at the locally advanced stage in China and western countries [2, 3]. To date, surgery offers the only potential curative treatment. With advances of peri-operative treatments and surgical techniques, the incidence of post-operative complications following gastrectomy has markedly declined. But infection still remains a common and sometimes deadly event causing higher hospital expenses and worse survival times [4,5,6,7,8].
The prognostic nutritional index (PNI), which was calculated from the albumin concentration and lymphocyte count, is a frequently used index to reflect the nutritional and immunological status of a patient, has been reported to be related to post-operative complications [9, 10]. Recently, emerging evidence has confirmed that malnutrition and suppressed systemic inflammation, which was assessed by PNI, was confirmed as an independent predictor for a poor prognosis for various types of cancer [9, 11,12,13,14]. Given infection was the most common complications and that previous studies have confirmed that malnutrition was adversely associated with post-operative outcomes [3, 9, 15], it seems reasonable to suppose that a low pre-operative PNI could predict infection after gastrectomy. Furthermore, considering that both malnutrition and infection weakens the immune system, and induces a pro-tumor environment [2, 16], an additive impact on prognosis may exist in GC patients having both a malnourished status and infection. However, this hypothesis needs to be rigorously investigated.
Here, we elucidated the impact of low PNI on post-operative infection, and the association among PNI, infection and prognosis of stage II or III GC patients following curative resection, by analyzing data of a high-volume institution in China. Furthermore, we explored whether peri-operative adjuvant chemotherapy (PAC, including both pre-operative and post-operative therapy) could counteract the impairment of a low PNI and infection for long-term outcomes.
Methods
Patients and data extraction
A total of 2577 patients with stage II or III gastric adenocarcinoma underwent curative gastrectomy (R0 resection and D2 lymphadenectomy) in the Hunan Cancer Hospital between November 2010 and December 2019. Of these 2577 patients, we excluded 35 with other synchronous cancers, 34 with remnants or recurrence of GC and 156 with acute infection before surgery, missing essential clinical, pathological, or follow-up data; the remaining 2352 patients were included in the subsequent analyses. Each patient signed informed consent for the operation and agreement to use their clinical data prior to surgery. Our retrospective study was authorized by the ethics committee of Hunan Cancer Hospital (No. 27 in 2021).
Peri-operative management and follow-up
All operations were performed or supervised by highly qualified surgeons. Digestive tract reconstruction and lymphadenectomy were based on the Japanese gastric cancer treatment guidelines and stages in the 8th edition of the TNM classification system [17, 18]. Although radical resection and post-operative adjuvant chemotherapy (AC) was applied to the overwhelming majority of patients with locally advanced GC in our institution, some patients with cT3-4/N + diseases received two to four cycles of neo-adjuvant chemotherapy (NAC) before surgery, using capecitabine/S-1 and oxaliplatin based regimens [17]. Usually, an open procedure was performed in stage II/III GC patients, but a few patients underwent total laparoscopic or laparoscopy-assisted gastrectomy. Prophylactic antibiotics (generally second-generation cephalosporins) were administered to all patients 30 min to 1 h before surgery and continued for 72 h post-operatively.
Post-operative morbidity and mortality within 30 days following surgery were confirmed by the Clavien–Dindo staging system [19]. Due to the lesser clinical importance of grade I complications, the present study analyzed only stage II or greater complications. Fluorouracil and platinum-based adjuvant chemotherapy (AC) was generally started about 28 days following surgery and maintained for about 6 months [20, 21].
Follow-up started 30 days after the operation, and once a quarter in the initial 2 years, every 6 months for the 3rd to 5th years and once a year thereafter. Physical and laboratory measurements were carried out at each follow-up. Ultrasonography or a CT scan was performed every 6 months during the initial 5 years post-operatively and endoscopy every 2 years. The last follow-up time was December 2020.
Evaluation
Clinicopathological data were collected from medical records and retrospectively analyzed. Laboratory measured were made 7 days prior to surgery, such as routine blood testing and albumin concentrations. The PNI score was calculated as the following equation: [serum albumin (g/L) + 0.005 × total lymphocyte count (/mm3)]. The cut-off value of PNI for overall survival (OS) was affirmed by X-tile, as described in previous study [11, 22]. Post-operative infectious complication (such as pulmonary infection, intra-abdominal infection) was diagnosed according to the Centers for Disease Control and Prevention as reported in previous study [3].
OS was defined as the operative day to death of any cause or the end of follow-up. To illustrate the potential addictive influence of low PNI and infection on survivals, patients were divided to four subgroups based on whether they had low PNI or developed infections. Patients who received ≥6 courses of PAC were assigned to the completion group, as patients providing <6 cycles of chemotherapy showed significantly worse prognosis [11, 20].
Statistical analysis
Data analyses were performed by SPSS software (ver. 24.0, IBM Corporation, New York, US). Measurement data were expressed as the mean ± SD or numbers (%). The difference between groups was investigated by chi-squared test with Fisher’s exact test or Student’s t-test (or a Mann–Whitney U test), as appropriate. Risk factors for infections were clarified using univariate and multivariate logistic regression analyses. The optimal cutoff PNI score for OS was confirmed using X-tile when reached the maximum χ2 log rank value. Survival data were compared by drafting a Kaplan–Meier curve and using the log rank test. Cox proportional hazard regression analysis using a forward conditional method was applied to estimate predictors that may influence OS. All statistical tests were bilateral, and P-value <0.05 was considered to be statistically significant.
Results
Patients’ Characteristics
Table 1 lists the basic characteristics of the entire 2352 patients. The majority of patients were male (66.5%), performed open procedure (75.0%), for a subtotal gastrectomy (71.9%), with stage III diseases (73.7%), and received PAC (72.2%). Body mass index (BMI) ranged from 13.84 to 37.18 kg/m2 with an average of 21.78 kg/m2. PNI ranged from 25.70 to 96.10 with an average of 47.47. The mean age, operation time, estimated intra-operative bleeding and post-operative hospital stay was 56.05 years (range, 19–86), 202 min (range, 64–584), 209 mL (range, 50–2300) and 10.9 days (range, 4–144), respectively. Additionally, 258 cases (11.0%) were performed NAC and 543 patients (23.1%) underwent blood transfusion peri-operatively.
The cutoff score of PNI for OS was 43.9 with a maximum χ2 log rank value of 19.3017 (Supplementary Fig. 1). Then, a total of 633 patients (26.9%) were diagnosed as poor nutritional and immunological status (PNI < 43.9). Comparison between groups found that a low PNI was associated with lower BMI, lymphocyte count, albumin, and hemoglobin concentrations, higher ASA score, more advanced tumor stage, higher frequency of blood transfusion, but less frequency of PAC (Table 1). Additionally, both post-operative infectious complications and severe complications were more common in patients with a low PNI.
Post-operative infections
Totally, 255 patients (10.8%) developed 346 adverse events in the initial 30 days after surgery, with 187 (54.0%) infections and 159 (46.0%) cases of non-infections, classified as Clavien–Dindo grade II or higher (Table 2). In particular, 160 patients developed 187 infections, involving 96 cases of intra-abdominal infections ranked first, followed by pneumonia (n = 73) and wound infections (n = 13). Additionally, 11 patients suffered from infection in those 258 patients (4.3%) who received NAC, which was comparable with those not received NAC (7.1%, P = 0.086).
Univariate analyses found that age ≥ 65 years, ASA score ≥ 3, BMI ≥ 25 kg/m2, comorbidity, pre-operative hemoglobin < 100 g/L, operation duration ≥ 240 min, intra-operative bleeding ≥ 300 mL and PNI < 43.9 was possible predictors for infection (P value < 0.05, Table 3a). Following multivariate regression analyses including the above mentioned parameters, operation duration ≥ 240 min, PNI < 43.9, comorbidity and BMI ≥ 25 kg/m2 were determined as independent predictors (Table 3b).
Predictors for poorer OS
The follow-up time in this study ranged from 1 to 122 months, with a median of 24 months. The median OS time was 56 months, and the median disease-free survival (DFS) time was 53 months. A total of 304 patients (48.0%) with PNI < 43.9 died during the follow-up time, which was significant more common than those with high PNI (38.0%, P < 0.001). Similarly, tumor recurrence occurred in 289 patients with low PNI (45.7%), which was more common compared to those with high PNI (651/1719, 37.9%, P = 0.001). Unsurprisingly, patients who received ≥ 6 cycles of PAC had significantly better survival than those receiving 1 to 5 cycles or without PAC (P < 0.001, Supplementary Fig. 2).
In the univariate analyses, BMI < 25 kg/m2, age ≥ 65 years, ASA score ≥ 3, PNI < 43.9, TNM stage III, blood transfusion, infections and without PAC showed potential relationship with poorer OS (P value < 0.05, Table 4). But subsequent multivariate analyses by enrolling the above mentioned variables determined that only stage III (hazard ratio (HR): 3.016, 95% confidence interval (CI): 2.509–3.627, P < 0.001), without PAC (HR: 1.610, 95% CI: 1.197–1.855, P < 0.001), infection (HR: 1.347, 95% CI: 1.067–1.700, P = 0.012), PNI < 43.9 (HR: 1.163, 95% CI: 1.007–1.343, P = 0.039) and blood transfusion (HR: 1.170, 95% CI: 1.006–1.361, P = 0.042) was significant predictors for poor OS.
Relationship between low PNI, infections and OS
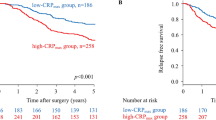
The median OS in those with higher PNI and not developed infection (the PNI ≥ 43.9/infection (−) group, n = 1617) was 68 months, which was significantly longer than 38, 29 and 19 months in the PNI < 43.9/infection (−) (n = 575), PNI ≥ 43.9/infection (+) (n = 101) and PNI < 43.9/infection (+) group (n = 59), respectively. In addition, a synergistic impact was found in the PNI < 43.9/infection (+) group, those patients with even worse prognosis compared to the PNI < 43.9/infection (−) or PNI ≥ 43.9/infection (+) group (P < 0.001) (Table 5, Fig. 1).
The median OS for the 687 cases receiving six or more cycles of PAC was not reached in the present study and significantly better than those with less than six cycles of PAC (43 months, n = 1665, P < 0.001). Further analyses confirmed that the OS time became comparable among the four groups, no matter PNI was high or low, or developing infections (P = 0.160, Fig. 2A). But four subgroups of patients with incomplete PAC had significantly different OS (P < 0.001, Fig. 2B), similarly as the entire cohort.
Discussion
This large cohort study demonstrated that not few patients (26.9%) with advanced stage of GC with poor immunological and nutritional status (defined as PNI < 43.9 in the present study), which was in consistence with other previous studies [15, 23]. Possible explanations for relatively higher incidence of malnutrition in locally advanced GC patients included the chronic inflammation, unplanned weight loss related to their cancer process and decreased dietary intake [9, 11, 24]. Serum albumin was the main plasma protein indicating nutritional status, and lymphocyte was the essential component of immune system to eradicate cancer cells. Thus, the PNI could be easily calculated from the albumin concentration and lymphocyte count to represent both the nutritional and immune-inflammatory host status, which might make it a superior index compared to other inflammation-based parameters, such as the neutrophil to lymphocyte ratio (NLR) or Glasgow prognostic score (GPS) [22, 25].
The association between PNI, morbidity and prognosis of GC has recently been investigated [9, 11, 12, 23, 26, 27] but the conclusions remain controversial. Hirahara et al. [12] conducted a retrospective study involving 368 patients who underwent minimally invasive surgery for GC, which revealed that post-operative complications were significantly related to PNI. There was also a multi-center study that reported that the PNI-low group (PNI < 47) experienced a higher incidence of morbidity, but whether low PNI was an independent predictor for complications has not been described [23]. In contrast, Sakurai and colleagues [26] did not find an association between PNI and post-operative morbidity in a cohort of 594 patients treated for GC by gastrectomy. This finding was echoed by Guner and colleagues [27], who argued that a low albumin concentration, instead of PNI, was a risk factor for major complications following curative gastrectomy in 1032 GC patients. The conflicting conclusions between research groups might be due to inconsistent criteria for patient enrollment and the relatively small sample sizes. In the present study, which involved 2352 patients, we demonstrated that a low PNI was a significant risk factor for infection in patients with stage II or III GC who underwent gastrectomy. The relatively larger sample size and the large number of examined variables could offer statistical power to improve the reliability of our conclusions.
With respect to prognosis, a low PNI was found to have an adverse impact on the long-term survival of stage II or III GC patients, a finding in keeping with previous studies [9, 11, 12, 23, 26]. In a meta-analysis conducted by Yang et al. [9], 10 studies involving 3396 GC patients were analyzed. They concluded that a low PNI was correlated with prognosis of stage I to III patients, but not stage IV. Although the exact reason has not been well established, a number of possible explanations have been proposed. First, malnutrition and a low lymphocyte count correlates to immunosuppression and thus provides a favorable micro-environment for tumor recurrence [9, 13]. Second, just as in similar previous studies, we found that low PNI was related to poor patients’ condition (such as a higher ASA score, more common comorbidities) and a more advanced tumor stage (Table 1), which are well-known predictors for prognosis in GC patients that impair survival. Third, as shown in Tables 1 and 3b, low PNI significantly increased the incidence of post-operative infection and was confirmed as an independent risk factor. Further analyses found that both low PNI and infection significantly affected survival and an additive impact was confirmed for the first time, based on the finding that patients who developed an infection and with a low PNI had the worst prognosis. The most likely explanation is that both malnutrition and infection induces inflammation and depresses host immunity, thus accelerating cancer cell proliferation and invasion [6, 10, 11, 13, 17, 28]. As malnutrition has an independent but also an additive effect with infection on long-term outcomes, to decrease infection and improve prognosis, peri-operative immunonutrition supplementation (such as omega-3 fatty acid, arginine, and nucleotide) seems to be a promise therapeutic strategy. Indeed, there is some clinical research, which has shown that immunonutrition reduced the risk of post-operative infectious complications [29, 30]. However, other investigators still failed to demonstrate any clear advantage [31, 32]. In addition, whether nutritional intervention improves survival in malnourished patients has not been fully clarified and needs further investigation.
Last but not least, as shown in Table 1, patients with low PNI seemed less likely to receive or complete peri-operative chemotherapy, as found in our previous study [13]. Our study involved 1288 stage II/III GC patients and PNI < 43.9 was confirmed to be a significant predictor of incomplete PAC. Given that PAC is considered to be the most common and important strategy to decrease tumor recurrence and thus improve prognosis for advanced stage GC after gastrectomy [13, 21], incompleteness of PAC might also partly explain the influence of low PNI on survival times. In the present study, the 5-year OS rate of patients having both low PNI and infection improved from 31.2% to 51.9% if they received six or more cycles of AC, which was comparable to patients with either low PNI or infection, or neither (P = 0.160, Fig. 2A). Thus, our conclusions strongly support PAC completion to improve survival in patients with a low PNI and/or infection, a finding echoed by Li and colleagues [33]. In the latter study, which involved a cohort of 206 patients with locally advanced GC who had undergone radical gastrectomy, the patients who suffered from major complications (Clavien–Dindo stage III or greater) were less likely to complete all planned multi-modality therapy. Further analysis found that completion of therapy could compensate for impairment of morbidity on prognosis. Other retrospective studies revealed that performing chemotherapy before surgery could increase the completion of PAC and was a feasible strategy to improve outcomes in patients developing morbidity after gastrectomy [10, 34]. But further prospective research with a larger sample size are still necessary to confirm this hypothesis.
The present study has a number of limitations. First, was its retrospective nature and being single center research. In addition, only Clavien–Dindo stage II or greater complications were analyzed, considering the relatively little clinical importance associated with grade I complications, which inevitably decreased the incidence of infection and thus might impair the reliability of our final conclusions. Second, although a growing number of evidence recommended NAC in patients with locally advanced GC [35], only 258 patients (11.0%) received NAC, and the relationship of response efficiency of NAC and prognosis has not been evaluated in the present study. Moreover, several chemotherapy regimens were used in our institution during the study, such as SOX, XELOX, ECF, FLOT4, et al. [8, 17, 20, 21, 35, 36]. This may also influence the completion of AC and prognosis, as a result, becoming a confounding factor and impair the generalizability of our conclusions. Third, the median follow-up time was only 2 years, which may be too short. However, the overwhelming majority of recurrences should occur within 24 months after resection [37] and in fact 940 patients (40.0%) relapsed during the follow-up period. Notwithstanding these limitations, our research nevertheless has clarified the association among nutrition, infection, and prognosis of stage II or III GC patients following radical gastrectomy, by studying a large cohort of patients.
In conclusion, this study from a high-volume institution in China identified that low PNI could independently predict post-operative infection in stage II/III GC patients who had undergone radical gastrectomy. Both low PNI and infections adversely impacted on survival times, and an additive impact was verified in patients who had low PNI and developed infections. Furthermore, receiving at least six cycles of peri-operative chemotherapy could cancer out the impairment of low PNI and infection on survival times. Thus, strategies to decrease the incidence of post-operative infection and to complete the planned chemotherapy is essential for stage II/III GC patients with low PNI to improve their survival times. Peri-operative immunonutrition supplementation and NAC seem to be possible approaches, but further prospective studies are required.
Data availability
The database used and/or analyzed during the current study is not publicly available (to maintain privacy) but can be available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-institution analysis of 765 patients from the US Gastric Cancer Collaborative. J Am Coll Surg. 2015;211:767–77.
Xiao H, Quan H, Pan S, Yin B, Luo W, Huang G, et al. Impact of peri-operative blood transfusion on post-operative infections after radical gastrectomy for gastric cancer: a propensity score matching analysis focusing on the timing, amount of transfusion and role of leukocyte depletion. J Cancer Res Clin Oncol. 2018;144:1143–54.
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with post-operative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83.
Endo S, Tsujinaka T, Fujitani K, Fujita J, Tamura S, Yamasaki M, et al. Risk factors for superficial incisional surgical site infection after gastrectomy: analysis of patients enrolled in a prospective randomized trial comparing skin closure methods. Gastric Cancer. 2016;19:639–44.
Kosuga T, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Konishi H, et al. Clinical and surgical factors associated with organ/space surgical site infection after laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2017;31:1667–74.
Xiao H, Xiao Y, Quan H, Liu W, Pan S, Ouyang Y. Intra-abdominal infection after radical gastrectomy for gastric cancer: Incidence, pathogens, risk factors and outcomes. Int J Surg. 2017;48:195–200.
Xiao H, Xiao Y, Chen P, Quan H, Luo J, Huang G. Association among blood transfusion, postoperative infectious complications, and cancer-specific survival in patients with stage II/III gastric cancer after radical gastrectomy: emphasizing benefit from adjuvant chemotherapy. Ann Surg Oncol. 2021;28:2394–404.
Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. 2016;42:1176–82.
Okada S, Shimada J, Teramukai S, Kato D, Tsunezuka H, Miyata N, et al. Risk stratification according to the prognostic nutritional index for predicting postoperative complications after lung cancer surgery. Ann Surg Oncol. 2018;25:1254–61.
Xiao H, Zhou H, Zhang P, Xiao H, Liu K, Chen X, et al. Association among the prognostic nutritional index, completion of adjuvant chemotherapy, and cancer-specific survival after curative resection of stage II/III gastric cancer. Eur J Clin Nutr. 2020;74:555–64.
Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. High preoperative prognostic nutritional index is associated with less postoperative complication-related impairment of long-term survival after laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2020;24:2852–5.
Noh GT, Han J, Cho MS, Hur H, Min BS, Lee KY, et al. Impact of the prognostic nutritional index on the recovery and long-term oncologic outcome of patients with colorectal cancer. J Cancer Res Clin Oncol. 2017;143:1235–42.
Ichikawa K, Mizuno S, Hayasaki A, Kishiwada M, Fujii T, Iizawa Y, et al. Prognostic nutritional index after chemoradiotherapy was the strongest prognostic predictor among biological and conditional factors in localized pancreatic ductal adenocarcinoma patients. Cancers. 2019;11:514.
Sun X, Xu J, Chen X, Zhang W, Chen W, Zhu C, et al. Sarcopenia in patients with normal body mass index is an independent predictor for postoperative complication and long-term survival in gastric cancer. Clin Transl Sci. 2021;14:837–46.
Xu J, Zhong Y, Jing D, Wu D. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30:1284–9.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1–21.
Amin MB, Edge SB, Greene FL, Brierley JD. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96.
Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, et al. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175–81.
Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–7.
Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer: analysis of a multi-institution dataset. Dig Surg. 2020;37:135–44.
Shen Q, Liu W, Quan H, Pan S, Li S, Zhou T, et al. Prealbumin and lymphocyte based prognostic score, a new tool for predicting long-term survival after curative resection of stage II/III gastric cancer. Br J Nutr. 2018;120:1359–69.
Imai D, Maeda T, Shimokawa M, Wang H, Yoshiya S, Takeishi K, et al. Prognostic nutritional index is superior as a predictor of prognosis among various inflammation-based prognostic scores in patients with hepatocellular carcinoma after curative resection. Hepatol Res. 2020;50:101–9.
Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, et al. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann Surg Oncol. 2016;23:525–33.
Guner A, Kim SY, Yu JE, Min IK, Roh YH, Roh C, et al. Parameters for predicting surgical outcomes for gastric cancer patients: simple is better than complex. Ann Surg Oncol. 2018;25:3239–47.
Salvans S, Mayol X, Alonso S, Messeguer R, Pascual M, Mojal S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260:939–43.
Zhang B, Wei G, Li R, Wang Y, Yu J, Wang R, et al. n-3 fatty acid-based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: A randomized controlled clinical trial. Clin Nutr. 2017;36:1239–44.
Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. 2013;20:3912–8.
Klek S, Kulig J, Sierzega M, Szybinski P, Szczepanek K, Kubisz A, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg. 2008;248:212–20.
Fujitani K, Tsujinaka T, Fujita J, Miyashiro I, Imamura H, Kimura Y, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99:621–9.
Li SS, Udelsman BV, Parikh A, Klempner SJ, Clark JW, Roeland EJ, et al. Impact of postoperative complication and completion of multimodality therapy on survival in patients undergoing gastrectomy for advanced gastric cancer. J Am Coll Surg. 2020;230:912–24.
Vicente D, Ikoma N, Chiang YJ, Fournier K, Tzeng CD, Song S, et al. Preoperative therapy for gastric adenocarcinoma is protective for poor oncologic outcomes in patients with complications after gastrectomy. Ann Surg Oncol. 2018;25:2720–30.
Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–92.
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–708.
Baiocchi GL, Marrelli D, Verlato G, Morgagni P, Giacopuzzi S, Coniglio A, et al. Follow-up after gastrectomy for cancer: an appraisal of the Italian research group for gastric cancer. Ann Surg Oncol. 2014;21:2005–11.
Acknowledgements
The authors gratefully thank all of the participants in this study and Hunan Cancer Hospital for supporting this study.
Funding
This study was supported by Hunan Provincial Natural Science Foundation of China (No. 2020JJ5339), Hunan Cancer Hospital Climb Plan (No. 2020NSFC-A004), the Science and Technology Foundation of Changsha (No. kq1907125), and the Research Project of Health Commission of Hunan Province (No. 20200608).
Author information
Authors and Affiliations
Contributions
HX, YPX, and GW contributed to the conception and the design of the study; HX, PYX, GW, MM, DL, PC, HQ, and JL collected, analyzed and interpreted the data; YPX and GW grafted the manuscript. HX critically revised the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approve the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, Y., Wei, G., Ma, M. et al. Association among prognostic nutritional index, post-operative infection and prognosis of stage II/III gastric cancer patients following radical gastrectomy. Eur J Clin Nutr 76, 1449–1456 (2022). https://doi.org/10.1038/s41430-022-01120-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01120-7