Abstract
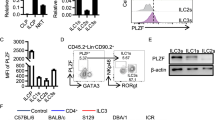
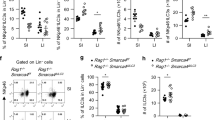
OX40L is one of the co-stimulatory molecules that can be expressed by splenic lymphoid tissue inducer (Lti) cells, a subset of group 3 innate lymphoid cells (ILC3s). OX40L expression in subsets of intestinal ILC3s and the molecular regulation of OX40L expression in ILC3s are unknown. Here, we showed intestinal ILC3s marked as an OX40Lhigh population among all the intestinal leukocytes and were the dominant source of OX40L in Rag1–/– mice. All ILC3 subsets expressed OX40L, and NCR–ILC3s were the most abundant source of OX40L. The expression of OX40L in ILC3s could be upregulated during inflammation. In addition to tumor necrosis factor (TNF)-like cytokine 1A (TL1A), which has been known as a trigger for OX40L, we found that Poly (I:C) representing viral stimulus promoted OX40L expression in ILC3s via a cell-autonomous manner. Furthermore, we demonstrated that IL-7-STAT5 signaling sustained OX40L expression by ILC3s. Intestinal regulatory T cells (Tregs), most of which expressed OX40, had defective expansion in chimeric mice, in which ILC3s were specifically deficient for OX40L expression. Consistently, co-localization of Tregs and ILC3s was found in the cryptopatches of the intestine, which suggests the close interaction between ILC3s and Tregs. Our study has unveiled the crosstalk between Tregs and ILC3s in mucosal tissues through OX40–OX40L signaling, which is crucial for the homeostasis of intestinal Tregs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Spits, H. & Cupedo, T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30, 647–675 (2012).
Diefenbach, A., Colonna, M. & Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41, 354–365 (2014).
Cording, S., Medvedovic, J., Cherrier, M. & Eberl, G. Development and regulation of RORgt(+) innate lymphoid cells. FEBS Lett. 588, 4176–4181 (2014).
Montaldo, E., Juelke, K. & Romagnani, C. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice. Eur. J. Immunol. 45, 2171–2182 (2015).
Zhang, Y. et al. Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation. Cell. Mol. Immunol. 15, 697–709 (2018).
van de Pavert, S. A. & Vivier, E. Differentiation and function of group 3 innate lymphoid cells, from embryo to adult. Int. Immunol. 28, 35–42 (2016).
Hepworth, M. R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
Hepworth, M. R. et al. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348, 1031–1035 (2015).
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Kim, M.-Y. et al. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity 18, 643–654 (2003).
Withers, D. R. et al. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J. Immunol. 189, 2094–2098 (2012).
Hatfield, J. K. & Brown, M. A. Group 3 innate lymphoid cells accumulate and exhibit disease-induced activation in the meninges in EAE. Cell. Immunol. 297, 69–79 (2015).
Chang, J. H. et al. The chronicity of tonsillitis is significantly correlated with an increase in an LTi cell portion. Inflammation 37, 132–141 (2014).
Kim, S. et al. CD117+ CD3− CD56− OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur. J. Immunol. 41, 1563–1572 (2011).
Kim, M. Y. et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J. Immunol. 174, 6686–6691 (2005).
Kim, M.-Y. et al. Neonatal and adult CD4+CD3− cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15). J. Immunol. 177, 3074–3081 (2006).
Croft, M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 (2010).
Chen, A. I. et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity 11, 689–698 (1999).
Griseri, T., Asquith, M., Thompson, C. & Powrie, F. OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 207, 699–709 (2010).
Rudensky, A. Y. Regulatory T cells and Foxp3. Immunol. Rev. 241, 260–268 (2011).
Chaudhry, A. & Rudensky, A. Y. Control of inflammation by integration of environmental cues by regulatory T cells. J. Clin. Invest. 123, 939–944 (2013).
Takeda, I. et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J. Immunol. 172, 3580–3589 (2004).
Kumar P., et al. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell. Mol. Immunol. 16, 138–153 (2019).
Korn, L. L. et al. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal Immunol. 7, 1045–1057 (2014).
Klose, C. S. et al. A T-bet gradient controls the fate and function of CCR6-RORgt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Sawa, S. et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 330, 665–669 (2010).
Eberl, G. et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Wirtz, S. et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 12, 1295–1309 (2017).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Pearson, C. et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife 5, e10066 (2016).
Longman, R. S. et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 211, 1571–1583 (2014).
Strober, W. & Fuss, I. J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140, 1756–1767 (2011).
Mills, K. H. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 11, 807–822 (2011).
Shih, D. Q. et al. Microbial induction of inflammatory bowel disease associated gene TL1A (TNFSF15) in antigen presenting cells. Eur. J. Immunol. 39, 3239–3250 (2009).
Ramnath, D., Powell, E. E., Scholz, G. M. & Sweet, M. J. The toll-like receptor 3 pathway in homeostasis, responses to injury and wound repair. Semin. Cell. Dev. Biol. 61, 22–30 (2017).
Levitzki, A. Targeting the immune system to fight cancer using chemical receptor homing vectors carrying polyinosine/cytosine (PolyIC). Front. Oncol. 2, 4 (2012).
Mosallanejad, K. et al. The DEAH-box RNA helicase DHX15 activates NF-kB and MAPK signaling downstream of MAVS during antiviral responses. Sci. Signal. 7, ra40 (2014).
Pobezinskaya, Y. L., Choksi, S., Morgan, M. J., Cao, X. & Liu, Z. G. The adaptor protein TRADD is essential for TNF-like ligand 1A/death receptor 3 signaling. J. Immunol. 186, 5212–5216 (2011).
Xiao, S. et al. Small-molecule RORgt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014).
Mulero, M. C. et al. Inhibiting the calcineurin-NFAT (nuclear factor of activated T cells) signaling pathway with a regulator of calcineurin-derived peptide without affecting general calcineurin phosphatase activity. J. Biol. Chem. 284, 9394–9401 (2009).
Song, H., Wang, R., Wang, S. & Lin, J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl Acad. Sci. USA 102, 4700–4705 (2005).
Leonard, W. J. et al. Signaling via the IL-2 and IL-7 receptors from the membrane to the nucleus. Cold Spring Harb. Symp. Quant. Biol. 64, 417–424 (1999).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Hanash, A. M. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012).
Goc, J. et al. Group 3 innate lymphoid cells: regulating host-commensal bacteria interactions in inflammation and cancer. Int Immunol. 28, 43–52 (2016).
Crellin, N. K. et al. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 33, 752–764 (2010).
Kim, K. D. et al. Adaptive immune cells temper initial innate responses. Nat. Med. 13, 1248–1252 (2007).
Kim, S. H., Cho, B. H., Kiyono, H. & Jang, Y. S. Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer's patches. Sci. Rep. 7, 3980 (2017).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014).
Xiao, X. et al. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J. Immunol. 181, 3193–3201 (2008).
So, T. & Croft, M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J. Immunol. 179, 1427–1430 (2007).
Zhang, X. et al. OX40 costimulation inhibits Foxp3 expression and Treg induction via BATF3-dependent and independent mechanisms. Cell Rep. 24, 607–618 (2018).
Vu, M. D. et al. OX40 costimulation turns off Foxp3+ Tregs. Blood 110, 2501–2510 (2007).
Ruby, C. E. et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J. Immunol. 183, 4853–4857 (2009).
Kim, K. S. et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351, 858–863 (2016).
Ohnmacht, C. et al. Mucosal immunology. The microbiota regulates type 2 immunity through RORgt+ T cells. Science 349, 989–993 (2015).
Halim, T. Y. F. et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 48, 1195–1207 e1196 (2018).
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39 (2014).
Wang, Y. et al. Discovery of biaryl amides as potent, orally bioavailable, and CNS penetrant RORgt inhibitors. ACS Med. Chem. Lett. 6, 787–792 (2015).
Acknowledgements
The authors would like to thank Dr. Dechun Feng for experimental support. We thank the entire Q.J. laboratory team for their help and suggestions. This study was supported by grants 2015CB943400 and 2014CB943300 from the Ministry of Science and Technology of China, grant XDB19000000 from the “Strategic priority research program of the Chinese Academy of Sciences”, grants 91542102 and 31570887 from the National Natural Science Foundation of China, and China's Youth 1000 Talent Program to Q.J.
Author information
Authors and Affiliations
Contributions
Q.J. and D.T. designed the research. D.T. and S.C. conducted the experiments and analyzed the data. Q.J. and D.T. wrote the manuscript. Y.R., C.J, L.J. and C.T. helped with the experiments and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, T., Suo, C., Chang, J. et al. ILC3-derived OX40L is essential for homeostasis of intestinal Tregs in immunodeficient mice. Cell Mol Immunol 17, 163–177 (2020). https://doi.org/10.1038/s41423-019-0200-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0200-x
Keywords
This article is cited by
-
Group 3 innate lymphoid cells in intestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2024)
-
Versatile roles of innate lymphoid cells at the mucosal barrier: from homeostasis to pathological inflammation
Experimental & Molecular Medicine (2023)
-
Reciprocal costimulatory molecules control the activation of mucosal type 3 innate lymphoid cells during engagement with B cells
Cellular & Molecular Immunology (2023)
-
Regulatory T cells in the face of the intestinal microbiota
Nature Reviews Immunology (2023)
-
Group 3 innate lymphoid cells: intestinal patrolling guardians bullied by T cells
Cellular & Molecular Immunology (2022)