Abstract
Background
Endocrine therapy is the mainstay treatment for breast cancer (BC) to reduce BC recurrence risk. During the first year of endocrine therapy use, nearly 30% of BC survivors are nonadherent, which may increase BC recurrence risk. This study is to examine the association between endocrine therapy adherence trajectories and BC recurrence risk in nonmetastatic BC survivors.
Methods
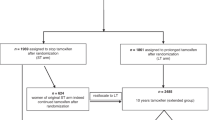
This retrospective cohort study included Medicare beneficiaries in the United States (US) with incident nonmetastatic BC followed by endocrine therapy initiation in 2010–2019 US Surveillance, Epidemiology, and End Results linked Medicare data. We calculated monthly fill-based proportion of days covered in the first year of endocrine therapy. We applied group-based trajectory models to identify distinct endocrine therapy adherence patterns. After the end of the first-year endocrine therapy trajectory measurement period, we estimated the risk of time to first treated BC recurrence within 4 years using Cox proportional hazards models.
Results
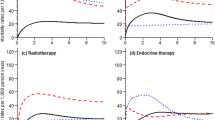
We identified 5 trajectories of adherence to endocrine therapy in BC Stages 0–I subgroup (n = 28,042) and in Stages II–III subgroup (n = 7781). A trajectory of discontinuation before 6 months accounted for 7.0% in Stages 0–I and 5.8% in Stages II–III subgroups, and this trajectory was associated with an increased treated BC recurrence risk compared to nearly perfect adherence (Stages 0–I: adjusted hazard [aHR] = 1.84, 95% CI = 1.46–2.33; Stages II–III: aHR = 1.38, 95% CI = 1.07–1.77).
Conclusions
Nearly 7% of BC survivors who discontinued before completing 6 months of treatment was associated with an increased treated BC recurrence risk compared to those with nearly perfect adherence among Medicare nonmetastatic BC survivors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
SEER-Medicare data are not publicly available. The data that support the findings of this study are available from the National Cancer Institute, but restrictions apply to the availability of these data, which are under license for the study, and so are not publicly available.
References
Surveillance, Epidemiology, and End Results Program National Center for Health Statistics. Cancer Stat Facts: Female breast cancer. 2019. https://seer.cancer.gov/statfacts/html/breast.html.
Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20:691–722.
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52.
Mansell J, Monypenny IJ, Skene AI, Abram P, Carpenter R, Gattuso JM, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2009;117:91–8.
Cheng L, Swartz MD, Zhao H, Kapadia AS, Lai D, Rowan PJ, et al. Hazard of recurrence among women after primary breast cancer treatment-a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomark Prev. 2012;21:800–9.
Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol. 2013;24:1443–9.
Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–9.
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66:43–73.
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–62.
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:936–42.
Dezentjé VO, van Blijderveen NJ, Gelderblom H, Putter H, van Herk-Sukel MP, Casparie MK, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28:2423–9.
Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol. 2013;36:181–7.
Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence, and mortality. Br J Cancer. 2013;108:1515–24.
Font R, Espinas JA, Barnadas A, Izquierdo A, Galceran J, Saladie F, et al. Influence of adherence to adjuvant endocrine therapy on disease-free and overall survival: a population-based study in Catalonia, Spain. Breast Cancer Res Treat. 2019;175:733–40.
Barron TI, Cahir C, Sharp L, Bennett K. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer. 2013;109:1513–21.
Lee Y, Park YR, Lee JS, Lee SB, Chung IY, Son BH, et al. Prescription refill gap of endocrine treatment from electronic medical records as a prognostic factor in breast cancer patients. J Breast Cancer. 2019;22:86–95.
McCowan C, Wang S, Thompson AM, Makubate B, Petrie DJ. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109:1172–80.
Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–50.
Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27:2015–23.
Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:Iv-3–18.
Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48.
Mues KE, Liede A, Liu J, Wetmore JB, Zaha R, Bradbury BD, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267–77.
Division of Cancer Control and Population Sciences. SEER-Medicare linked data resource. 2023. https://healthcaredelivery.cancer.gov/seermedicare/.
Health Resources and Services Administration. Area health resources files. 2022. https://data.hrsa.gov/.
Wei YJ, Palumbo FB, Simoni-Wastila L, Shulman LM, Stuart B, Beardsley R, et al. Antiparkinson drug use and adherence in medicare part D beneficiaries with Parkinson’s disease. Clin Ther. 2013;35:1513–25.e1511.
Wei YJ, Simoni-Wastila L, Albrecht JS, Huang TY, Moyo P, Khokhar B, et al. The association of antidepressant treatment with COPD maintenance medication use and adherence in a comorbid Medicare population: a longitudinal cohort study. Int J Geriatr Psychiatry. 2018;33:e212–20.
Dong YH, Choudhry NK, Krumme A, Lee MP, Wu LC, Lai MS, et al. Impact of hospitalization on medication adherence estimation in claims data. J Clin Pharm Ther. 2017;42:318–28.
Michaelson JS, Halpern E, Kopans DB. Breast cancer: computer simulation method for estimating optimal intervals for screening. Radiology. 1999;212:551–60.
Nau D. Pharmacy quality alliance adherence measures. 2019. https://www.pqaalliance.org/adherence-measures.
Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12.
Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15:457–64.
Chang AA. SAS macro to calculate the PDC adjustment of inpatient stays. SAS Global Forum Proceedings. 2015. https://support.sas.com/resources/papers/proceedings15/3560-2015.pdf
Centers for Medicare & Medicaid Services. Physician Quality Reporting System Measures Specifications Manual for Claims and Registry Reporting of Individual Measures. Baltimore: CMS; 2011.
Schwarz G. Estimating the dimension of a model. Ann Stat. 1978:461–4.
Chubak J, Yu O, Pocobelli G, Lamerato L, Webster J, Prout MN, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst. 2012;104:931–40.
Winn AN, Dusetzina SB. The association between trajectories of endocrine therapy adherence and mortality among women with breast cancer. Pharmacoepidemiol Drug Saf. 2016;25:953–9.
Chien HC, Kao Yang YH, Kwoh CK, Chalasani P, Wilson DL, Lo-Ciganic WH. Aromatase inhibitors and risk of arthritis and carpal tunnel syndrome among taiwanese women with breast cancer: a nationwide claims data analysis. J Clin Med. 2020;9:566.
Mausbach BT, Schwab RB, Irwin SA. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015;152:239–46.
Haskins CB, McDowell BD, Carnahan RM, Fiedorowicz JG, Wallace RB, Smith BJ, et al. Impact of preexisting mental illness on breast cancer endocrine therapy adherence. Breast Cancer Res Treat. 2019;174:197–208.
Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–72.
Mieog JSD, Morden JP, Bliss JM, Coombes RC, van de Velde CJ, Committee IS. Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2–3 years of tamoxifen: a retrospective analysis of the Intergroup Exemestane Study. lancet Oncol. 2012;13:420–32.
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–83.
Beckwée D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer. 2017;25:1673–86.
Wigertz A, Ahlgren J, Holmqvist M, Fornander T, Adolfsson J, Lindman H, et al. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast cancer Res Treat. 2012;133:367–73.
Farias AJ, Hansen RN, Zeliadt SB, Ornelas IJ, Li CI, Thompson B. Factors associated with adherence to adjuvant endocrine therapy among privately insured and newly diagnosed breast cancer patients: a quantile regression analysis. J Manag Care Spec Pharm. 2016;22:969–78.
Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharm Ther. 2011;90:377–87.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–2.
Guo S, Fraser MW. Propensity score analysis: statistical methods and applications, Vol. 11. SAGE Publications; 2014.
Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26:1654–70.
Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circulation Cardiovasc Qual Outcomes. 2013;6:604–11.
Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution-a simulation study. Am J Epidemiol. 2010;172:843–54.
Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26.
Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–8.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74.
Warren JL, Mariotto A, Melbert D, Schrag D, Doria-Rose P, Penson D, et al. Sensitivity of medicare claims to identify cancer recurrence in elderly colorectal and breast cancer patients. Med Care. 2016;54:e47–54.
Hassett MJ, Ritzwoller DP, Taback N, Carroll N, Cronin AM, Ting GV, et al. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med Care. 2014;52:e65–73.
Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51:789–96.
Lo-Ciganic WH, Donohue JM, Jones BL, Perera S, Thorpe JM, Thorpe CT, et al. Trajectories of diabetes medication adherence and hospitalization risk: a retrospective cohort study in a large state Medicaid program. J Gen Intern Med. 2016;31:1052–60.
Lo-Ciganic WH, Gellad WF, Gordon AJ, Cochran G, Zemaitis MA, Cathers T, et al. Association between trajectories of buprenorphine treatment and emergency department and inpatient utilization. Addiction. 2016;111:892–902.
Lo-Ciganic WH, Donohue JM, Kim JY, Krans EE, Jones BL, Kelley D, et al. Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiol Drug Saf. 2019;28:80–89.
Acknowledgements
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Author information
Authors and Affiliations
Contributions
Chang and Lo-Ciganic had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Chang, Jones, Hincapie-Castillo, Park, Heldermon, Lo-Ciganic. Acquisition of data: Chang and Lo-Ciganic. Analysis and interpretation of data: Chang, Jones, Hincapie-Castillo, Park, Heldermon, Diaby, Wilson, Lo-Ciganic. Drafting of the manuscript: Chang and Lo-Ciganic. Critical revision of the manuscript for important intellectual content: None. Statistical analysis: Chang, Jones, and Lo-Ciganic. Obtained funding: None. Administrative, technical, or material support: Jones, Wilson, and Lo-Ciganic. Study supervision: Lo-Ciganic.
Corresponding author
Ethics declarations
Competing interests
W.-H.L.-C. reported receiving grants from the National Institute on Drug Abuse, the National Institute on Aging, the National Institute of Mental Health, Merck Sharp & Dohme, Bristol Myers Squibb, the Richard King Mellon Foundation at the University of Pittsburgh, the Clinical and Translational Science Institute of the University of Florida, the Pharmaceutical Research and Manufacturers of America Foundation, and the US Department of Veterans Affairs outside the submitted work; and having a patent pending for U1195.70174US00. H.P. reported receiving grants from Bristol Myers Squibb/Pfizer Alliance American Thrombosis Investigator Initiated Research Program outside the submitted work. J.M.H.-C. reported receiving grants from Merck & Co outside the submitted work. D.L.W. reported receiving grants from the National Institute on Drug Abuse, the National Institute on Aging, Merck Sharp & Dohme, and Bristol Myers Squibb outside the submitted work; and serving as an editorial board member for the Journal of Pharmacy Technology. C.-Y.C. contributions to this manuscript were made while at the University of Florida College of Pharmacy. C.-Y.C. is currently employed by Vertex Pharmaceuticals, Inc. Vertex did not fund or have any involvement in this study or publication. V.D. is currently employed by Otsuka, Inc. Otsuka did not fund or have any involvement in this study or publication. No other disclosures were reported.
Ethics approval and consent to participate
This study was approved by the institutional review board at the University of Florida, which waived the need for obtaining informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chang, CY., Jones, B.L., Hincapie-Castillo, J.M. et al. Association between trajectories of adherence to endocrine therapy and risk of treated breast cancer recurrence among US nonmetastatic breast cancer survivors. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02680-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02680-0