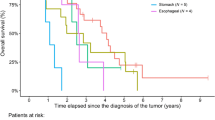
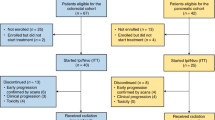
Abstract
Background
The outstanding efficacy of immunotherapy in metastatic dMMR/MSI gastro-intestinal (GI) cancers has led to a rapid increase in the number of patients treated. However, 20-30% of patients experience primary resistance to immune checkpoint inhibitors (ICIPR) and need better characterization.
Methods
This AGEO real-world study retrospectively analyzed the efficacy and safety of ICIs and identified clinical variables associated with ICIPR in patients with metastatic dMMR/MSI GI cancers treated with immunotherapy between 2015 and 2022.
Results
399 patients were included, 284 with colorectal cancer (CRC) and 115 with non-CRC, mostly treated by an anti-PD(L)1 (88.0%). PFS at 24 months was 55.8% (95CI [50.8–61.2]) and OS at 48 months was 59.1% (95CI [53.0–65.9]). ORR was 51.0%, and 25.1% of patients were ICIPR. There was no statistical difference in ORR, DCR, PFS, or OS between CRC and non-CRC groups. In multivariable analysis, ICIPR was associated with ECOG-PS ≥ 2 (OR = 3.36), liver metastases (OR = 2.19), peritoneal metastases (OR = 2.00), ≥1 previous line of treatment (OR = 1.83), and age≤50 years old (OR = 1.76).
Conclusion
These five clinical factors associated with primary resistance to ICIs should be considered by physicians to guide treatment choice in GI dMMR/MSI metastatic cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
GLOBOCAN 2020.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer). N. Engl J Med. 2005;352:1851–60.
Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, et al. Correlation between density of CD8+ T-cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 2015;75:3446–55.
Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: diagnosis, prognosis and treatment. Eur J Cancer. 2022;175:136–57.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1–10.
Taïeb J, Sayah L, Heinrich K, Kunzmann V, Boileve A, Cirkel G, et al. Efficacy of immune checkpoint inhibitors in microsatellite unstable/mismatch repair-deficient advanced pancreatic adenocarcinoma: an AGEO European Cohort. Eur J Cancer. 2023;188:90–7.
André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl J Med. 2020;383:2207–18.
Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:665–77.
Taïeb J, Bouche O, André T, Le Malicot K, Laurent-Puig P, Bez J, et al. Avelumab vs Standard Second-Line Chemotherapy in Patients With Metastatic Colorectal Cancer and Mi-crosatellite Instability: A Randomized Clinical Trial. JAMA Oncol. 2023;9:1356–63.
Keytruda authorisation details [Internet]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda#authorisation-details-section
Yervoy details [Internet]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/yervoy
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl J Med. 2015;372:2509–20.
Overman MJ, Bergamo F, McDermott RS, Aglietta M, Chen F, Gelsomino F, et al. Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): Long-term survival according to prior line of treatment from CheckMate-142. J Clin Oncol. 2018;36:554–554.
Le DT, Kavan P, Kim TW, Burge ME, Van Cutsem E, Hara H, et al. KEYNOTE-164: Pembrolizumab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J Clin Oncol. 2018;36:3514–3514.
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus Ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9.
Rousseau B, Foote MB, Maron SB, Diplas BH, Lu S, Argilés G, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N. Engl J Med. 2021;384:1168–70.
Loupakis F, Depetris I, Biason P, Intini R, Prete AA, Leone F, et al. Prediction of benefit from checkpoint inhibitors in mismatch repair deficient metastatic colorectal cancer: role of tumor infiltrating lymphocytes. Oncologist. 2020;25:481–7.
Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30:1232–43.
FDA approval combination therapy [Internet]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-ipilimumab-msi-h-or-dmmr-metastatic-colorectal-cancer
Cohen R, Hain E, Buhard O, Guilloux A, Bardier A, Kaci R, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol. 2019;5:551–5.
Mazzoli G, Cohen R, Lonardi S, Corti F, Elez E, Fakih M, et al. Prognostic impact of performance status on the outcomes of immune checkpoint inhibition strategies in patients with dMMR/MSI-H metastatic colorectal cancer. Eur J Cancer. 2022;172:171–81.
Pietrantonio F, Loupakis F, Randon G, Raimondi A, Salati M, Trapani D, et al. Efficacy and safety of immune checkpoint inhibitors in patients with microsatellite instability-high end-stage cancers and poor performance status related to high disease burden. Oncologist. 2020;25:803–9.
Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, Nagashima K, et al. Pretreatment immune status correlates with progression-free survival in chemotherapy-treated metastatic colorectal cancer patients. Cancer Immunol Res. 2016;4:592–9.
Sargent DJ, Köhne CH, Sanoff HK, Bot BM, Seymour MT, de Gramont A, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:1948–55.
Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–19.
Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32.
Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17:3173–80.
Rijken A, Bakkers C, van Erning FN, van der Geest LG, de Vos-Geelen J, Besselink MG, et al. Incidence, treatment, and survival of synchronous peritoneal metastases in pancreatic cancer: update of a nationwide cohort. Pancreas. 2021;50:827–33.
Donnenberg AD, Luketich JD, Dhupar R, Donnenberg VS. Treatment of malignant pleural effusions: the case for localized immunotherapy. J Immunother Cancer. 2019;7:110.
Chow A, Schad S, Green MD, Hellmann MD, Allaj V, Ceglia N, et al. Tim-4+ cavity-resident macrophages impair anti-tumor CD8+ T cell immunity. Cancer Cell. 2021;39:973–988.e9.
Fucà G, Cohen R, Lonardi S, Shitara K, Elez ME, Fakih M, et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J Immunother Cancer. 2022;10:e004001.
Huyghe N, Benidovskaya E, Stevens P, Van den Eynde M. Biomarkers of response and resistance to immunotherapy in microsatellite stable colorectal cancer: toward a new personalized medicine. Cancers. 2022;14:2241.
Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with Anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–24.
Saberzadeh-Ardestani B, Jones JC, Hubbard JM, McWilliams RR, Halfdanarson TR, Shi Q, et al. Association between survival and metastatic site in mismatch repair-deficient metastatic colorectal cancer treated with first-line Pembrolizumab. JAMA Netw Open. 2023;6:e230400.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–64.
Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5:eaba0759.
Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612:141–7.
Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–41.
Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front Immunol. 2020;11:369.
Chang, Pai DT, Rybicki RK, Dimaio MA LA, Limaye M, Jayachandran P, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–39. Aug
Ansari D, Althini C, Ohlsson H, Andersson R. Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch Surg. 2019;404:565–71.
Liu H, Li Z, Zhang Q, Li Q, Zhong H, Wang Y, et al. Multi‑institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front Immunol. 2022;13:1007176.
Cona M, Lecchi M, Cresta S, Damian S, Del Vecchio M, Necchi A, et al. Combination of baseline LDH, performance status and age as integrated algorithm to identify solid tumor patients with higher probability of response to Anti PD-1 and PD-L1 monoclonal antibodies. Cancers. 2019;11:223.
Wu Q, Wang Q, Tang X, Xu R, Zhang L, Chen X, et al. Correlation between patients’ age and cancer immunotherapy efficacy. Oncoimmunology 2019;8:e1568810.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13.
Colle R, Lonardi S, Cachanado M, Overman MJ, Elez E, Fakih M, et al. BRAF V600E/RAS Mutations and Lynch Syndrome in Patients With MSI-H/dMMR Metastatic Colorectal Cancer Treated With Immune Checkpoint Inhibitors. Oncologist. 2023 Apr 6;oyad082.
Acknowledgements
We would like to thank AGEO and David Marsh for supporting the study and English language correction, respectively. We thank the study staff for their contributions as well as Prof Frederic Di Fiore and Prof Stefano Kim.
Author information
Authors and Affiliations
Contributions
Conceptualization: JT, DT and RG. Data curation: CF, EAl and MM. Formal analysis: CF, EAu and JT. Methodology: CF, EAu, and JT. Validation: JT. Writing, review, and editing: CF, JT, EAu, DT and RG. Validation of final manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
TM has received honoraria as a speaker or in an advisory role from Servier, Pierre Fabre, Merck Serono, AAA, Sanofi, and research funding from Amgen; and travel grants from Pierre Fabre, Merck Serono, and Sanofi. RC has received honoraria from AstraZeneca, Bristol-Myers Squibb, MSD Oncology, Mylan Medical, Pierre Fabre, Servier, and travel/accommodation fees from Amgen, Bristol-Myers Squibb, Mylan Medical, and Servier. CB has received honoraria from Bayer, Pierre Fabre, and MSD and research grants from Bayer and Roche. FS has received honoraria for consultancy, advisory roles, or speeches from AMAL Therapeutics, Amgen, Bayer, BMS, Dragonfly Therapeutics, Merck, Nordic Pharma, Roche, and Servier, research funding (institute) from Amgen, Astra Zeneca, Bayer, BMS, Roche, and Sanofi, support for travel/accommodation from Amgen, Bayer, Lilly, and Servier, and serves as Co-Chair of the EORTC Task Force Colon, Rectum, Anal Canal. TA has received honoraria as a speaker or in an advisory role from Amgen, Servier, Pierre Fabre, BMS, SIRTEC, and MSD. CG has participated in consulting and/or advisory boards for Servier, Sanofi, Merck, and Pierre Fabre, and has received support for travel to meetings from Amgen and Pierre Fabre. DT has received honoraria as a speaker or in an advisory role from Sanofi, Roche, Merck Serono, Amgen, Servier, Pierre Fabre, BMS, Astra Zeneca, and MSD. JT has received honoraria as a speaker or in an advisory role from Sanofi, Roche, Merck Serono, Amgen, Servier, Pierre Fabre, Lilly, Astra Zeneca, and MSD. Other authors do not report any competing interest.
Ethics approval and consent to participate
This study was approved by the French Data Protection Authority (CNIL) (2222018v0) and by the APHP (Assistance Publique des Hopitaux de Paris) ethics and research committee. It complies with the Declaration of Helsinki and is registered on the Health Data Hub site No. F20210315164546 within the framework of the MR-004 methodology. An information memo was given to all centers to be sent to patients. No patient objected to the use of their data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flecchia, C., Auclin, E., Alouani, E. et al. Primary resistance to immunotherapy in patients with a dMMR/MSI metastatic gastrointestinal cancer: who is at risk? An AGEO real-world study. Br J Cancer 130, 442–449 (2024). https://doi.org/10.1038/s41416-023-02524-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02524-3