Abstract
Background
Tumour-associated fat cells without desmoplastic stroma reaction at the invasion front (Stroma AReactive Invasion Front Areas (SARIFA)) is a prognostic biomarker in gastric and colon cancer. The clinical utility of the SARIFA status in oesophagogastric cancer patients treated with perioperative chemotherapy is currently unknown.
Methods
The SARIFA status was determined in tissue sections from patients recruited into the MAGIC (n = 292) or ST03 (n = 693) trials treated with surgery alone (S, MAGIC) or perioperative chemotherapy (MAGIC, ST03). The relationship between SARIFA status, clinicopathological factors, overall survival (OS) and treatment was analysed.
Results
The SARIFA status was positive in 42% MAGIC trial S patients, 28% MAGIC and 48% ST03 patients after pre-operative chemotherapy. SARIFA status was related to OS in MAGIC trial S patients and was an independent prognostic biomarker in ST03 trial patients (HR 1.974, 95% CI 1.555–2.507, p < 0.001). ST03 patients with lymph node metastasis (ypN + ) and SARIFA-positive tumours had poorer OS than patients with ypN+ and SARIFA-negative tumours (plogrank < 0.001).
Conclusions
The SARIFA status has clinical utility as prognostic biomarker in oesophagogastric cancer patients irrespective of treatment modality. Whilst underlying biological mechanisms warrant further investigation, the SARIFA status might be used to identify new drug targets, potentially enabling repurposing of existing drugs targeting lipid metabolism.
Similar content being viewed by others
Introduction
Gastric cancer is ranked as the fifth most common cancer worldwide accounting for ~769,000 cancer-associated deaths in 2020 [1]. The introduction of perioperative or neoadjuvant combination chemotherapy significantly improved the outcome in patients with tumour-node-metastasis (TNM) stage II or III gastric or oesophagogastric cancers [2]. The greatest benefit from perioperative combination chemotherapy seems to come from the preoperative part as in most trials, including MAGIC and ST03, a significant number of patients did not complete the postoperative treatment as originally planned in the protocol. Despite this progress, death due to locally recurrent disease or distant metastasis remains a major challenge [3]. In everyday clinical practice, the clinical decision on the postoperative treatment and surveillance strategy is highly relevant with regard to tolerability and quality of life. Therefore, there remains an urgent clinical need to identify a biomarker which can predict the risk of recurrent disease and/or overall survival (OS) after neoadjuvant therapy and surgical resection in order to personalise postoperative follow-up and treatment.
Histomorphological biomarker such as tumour budding [4] or tumour-stroma ratio [5], as well as a several molecular classifications have been proposed to predict prognosis or response to therapy in oesophagogastric cancer patients [6, 7]. However, to date, none of these has been introduced into routine clinical practice and TNM disease stage continues to be the only clinically used prognostic parameter informing treatment decision in oesophagogastric cancer patients.
We previously identified tumour-associated fat cells without desmoplastic stroma reaction at the invasion front (Stroma Areactive Invasion Front Areas (SARIFA)) as a new histomorphological prognostic biomarker in patients with gastric or colon cancer from a single hospital [8, 9]. We demonstrated that the SARIFA status (positive vs negative) can be determined on routine Haematoxylin & Eosin (HE) stained tissue sections with high interobserver agreement. Moreover, we provided the first evidence for an interaction between adipocytes and tumour cells using digital spatial profiling suggesting that SARIFA-positive tumours may have an altered immune response (Fig. 1).
a Usually, tumour cell invasion induces a desmoplastic stroma reaction at the invasive edge. Inflammatory cells (mostly lymphocytes) are seen close to tumour cells in the stroma. Cancer-associated fibroblasts proliferate, producing extracellular matrix such as collagen, whereby the stroma becomes more dense and less cellular over time. b In SARIFA-positive tumours, there is at least focally no desmoplastic reaction at all. The host’s adipocytes are located directly adjacent to the tumour cells and a direct interaction between tumour cells and adipocytes seems possible to the advantage of the tumour. c Crosstalk between tumour cells, adipocytes and macrophages may induce a change in the metabolism of the tumour cells resulting in several tumour promoting effects. Tumour-associated fat cells may serve as an exogenous source of fatty acids. Fatty acid binding proteins (FABP) seem to be of particular importance with a supposed function for the intracellular lipid transport to specific compartments [20,21,22]. Our own findings suggest that FABP4 is upregulated in tumour cells of SARIFA-positive gastric cancers with concomitant increased CD36 (fatty acid translocase) protein expression. Adipocytes promote MII polarisation of macrophages which may lead to immune suppression [23]. The crosstalk may therefore promote tumour cell survival, proliferation, migration, invasion, and angiogenesis.
Based on these initial observations, we hypothesised that patients with SARIFA-positive oesophagogastric cancer have a poor prognosis irrespective whether they have been treated with surgery alone or perioperative combination chemotherapy.
The aim of the study was to establish whether the SARIFA status has clinical utility as a predictive and/or prognostic biomarker in oesophagogastric cancer patients with locally advanced resectable disease. Therefore, we determined the SARIFA status in HE stained tissue sections from resection specimens of 985 patients recruited into the United Kingdom Medical Research Council (MRC) Adjuvant Gastric Infusional Chemotherapy (MAGIC) and UK MRC ST03 randomised trials and investigated the relationship between SARIFA status, clinicopathological variables, OS and treatment.
Material and methods
MAGIC and ST03 trial patients
The MAGIC trial was an open-label, multicenter, phase 3 clinical trial randomising patients with resectable oesophagogastric adenocarcinoma to either perioperative epirubicin, cisplatin, and 5-fluorouracil chemotherapy plus surgery (ECF) or surgery alone (S) between July 1994 and April 2002. Of the 503 randomised patients, 453 (90%) patients proceeded to surgical resection. For a previous study, a single representative Haematoxylin/Eosin (HE) stained tissue section with tumour was collected and scanned at 40x magnification (Leica Aperio AT scanner, University of Leeds, Leeds, UK). Slides from 335 resection specimens were available for review for the purpose of the current study. Patients with complete pathological response were not included in the current study.
The UK MRC ST03 clinical trial was an open-label, multicenter, phase 3 clinical trial randomising 1063 patients with resectable gastroesophageal adenocarcinoma to either perioperative epirubicin, cisplatin, and capecitabine (ECX) chemotherapy or ECX plus Bevacizumab (ECX-Bev) between October 2007 and March 2015 [10]. Of these, 903 (85%) patients proceeded to surgical resection. During the trial, 20,277 slides from 799 resection specimens were collected prospectively and scanned at 40x magnification (Leica Aperio AT scanner, University of Leeds, Leeds, UK). Slides from ST03 patients with complete pathological response (n = 56) or those taking part in the Lapatinib feasibility substudy (n = 6, 0.8%) were excluded from analyses. HE stained tissue sections from pretreatment (diagnostic) endoscopic biopsies of 100 randomly selected patients were also included in this study.
Individual patient clinicopathological characteristics (sex, age, body mass index (BMI), depth of invasion (y)pT, lymph node status (y)pN, primary tumour regression grade, tumour localisation and resection margin status), and outcome data were retrieved from MRC Clinical Trial Unit London (UK) databases, original histopathology reports or established during review of scanned slides.
This study was approved by the UK NRES Committee London-South East (IRAS ID 144220).
SARIFA status assessment
The SARIFA status was determined reviewing all available digital HE stained resection specimen sections from both trials². In the MAGIC trial, a single representative tumour section was available per patient only, whereas in the ST03 trial, multiple slides with primary tumour were available from most of the resection specimen. SARIFA positivity was defined as presence of an area within the primary tumour where at least a single tumour gland or a group of at least five tumour cells are located directly adjacent to adipocytes. In the normal wall of the stomach or oesophagus, adipocytes are most commonly seen in the subserosa, but can also be present in the submucosa or around large vessels within the wall. Presence of one area classified as SARIFA-positive was sufficient to classify a whole tumour as SARIFA-positive, size of the area was not considered. If SARIFA-positive areas were only observed in one of the tumour containing slides in the ST03 trial, the number of tumour cells, SARIFA areas and adipocytes involved in the SARIFA area were noted. If there was no SARIFA-positive area in any of the slides, the whole tumour was classified as SARIFA-negative. Cases with complete pathological regression (e.g. no tumour on any of the slides), intramucosal cancer only, poor slide quality or other technical problems were classified as non-assessable. The assessment rules were the same for all resection slides irrespective of the type of treatment.
In addition, 100 pretreatment endoscopic biopsies from ST03 patients were screened for the presence of fat to assess feasibility of determining SARIFA status in pretreatment biopsies.
All slides were assessed by one observer (BG) blinded to any clinicopathological information. A second observer (HG) reviewed all the cases that were deemed as not-assessable by the first observer (MAGIC: n = 21 (6%), ST03: n = 44 (6%)).
Statistical analyses
The relationship between the SARIFA status and clinicopathological characteristics (primary tumour regression grade according to Mandard [11], sex, depth of invasion ((y)pT), lymph node status ((y)pN), resection margin (R) status, treatment, tumour location, BMI) was analysed using the Kruskal-Wallis test, Mann-Whitney U test or Chi square test as appropriate. Analyses were stratified by treatment arm in the MAGIC trial as we were interested in comparing the relationship between the SARIFA status and survival in surgery alone treated patient with that of patients treated with perioperative chemotherapy. As patients in both treatment arms in ST03 received perioperative chemotherapy and the initial analysis showed that the relationship between the SARIFA status and survival was similar in both treatment arms, we did not stratify the analyses by treatment in ST03. In the ST03 trial, we additionally classified patients combining the SARIFA status and the lymph node status into (a) SARIFA-positive + ypN0, (b) SARIFA-positive + ypN+ (N+: presence of lymph node metastasis irrespective of number of metastases), (c) SARIFA-negative + ypN0 and (d) SARIFA-negative + ypN+ to analyse the relationship of this combined classifier with OS.
The primary outcome measure was OS from the date of randomisation with surviving patients censored at their date of last follow-up. For univariable survival analyses,
Kaplan–Meier estimates were compared using log-rank tests, and hazard ratio (HR)s were calculated from Cox proportional hazard models. Multivariable analysis was performed using a Cox proportional hazard model including known prognostic factors ypT, ypN, ypR, and Mandard TRG.
Statistical analyses were performed using SPSS (Version 28, IBM Corp., Armonk, NY, USA) and R Version 4.2.1.
Results
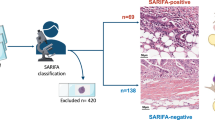
SARIFA status was evaluated in primary tumour slides from 335 patients in the MAGIC trial and 799 patients in the ST03 trial. Example images for SARIFA-positive and SARIFA-negative areas are presented in Fig. 2.
a SARIFA-positive cancer from a surgery alone treated MAGIC trial patient showing tumour cells directly adjacent to adipocytes without desmoplastic stroma reaction (scale bar 200 µm; inserts 50 µm); a1: overview, a2 inset 1: showing several large fat cells (optically empty spaces) directly adjacent to tumour cells. a3 inset 2: tumour cells and fat cells annotated. b SARIFA-positive cancer from a ST03 trial patient after neoadjuvant chemotherapy showing extensive fat in the subserosa, no desmoplastic stroma reaction (scale bar 200 µm; inserts 50 µm). b1: overview, b2 inset 1: showing several large fat cells (optically empty spaces) directly adjacent to tumour cells. b3 inset 2: tumour cells and fat cells annotated. c SARIFA-positive cancer from a ST03 trial patient after neoadjuvant chemotherapy showing fat adjacent to tumour cells in the submucosa, no desmoplastic stroma reaction (scale bar 500 µm; inserts 50 µm). c1: overview, c2 inset 1: showing several large fat cells (optically empty spaces) directly adjacent to tumour cells. c3 inset 2: with annotated tumour cells and fat cells. d SARIFA-negative cancer from a surgery alone treated MAGIC trial patient showing a desmoplastic stroma reaction between tumour and fat at the invasive front (scale bar 500 µm; insert 200 µm). d1: overview, d2: tumour cells, fat cells, and desmoplastic stroma annotated. e SARIFA-negative cancer from a ST03 trial patient after neoadjuvant chemotherapy showing desmoplastic stroma with inflammation between tumour and fat at the invasive front (scale bar 200 µm; insert 50 µm). e1: overview, e2: tumour cells, fat cells and desmoplastic stroma with inflammation annotated.
The SARIFA status was classified as non-assessable in 43 (12.8%) and 106 (13.3%) tumours in the MAGIC trial and ST03 trial, respectively, leaving 292 patients from the MAGIC trial (158 (54.1%) S patients, 134 (45.9%) ECF patients) and 693 patients from the ST03 trial (364 (52.5%) ECX patients, 329 (47.5%) ECX +Bev patients) for final analyses, see Supplementary Fig. S1.
In the MAGIC trial, 103 (35.3%) tumours were classified SARIFA-positive. The frequency of SARIFA-positive tumours was significantly higher in S patients compared to ECF patients (S: n = 66, 41.8%, ECF: n = 37, 27.6%, p = 0.012). Therefore, all subsequent analyses in the MAGIC trial were stratified by treatment arm.
In the ST03 trial, 336 (48.5%) tumours were classified SARIFA-positive (ECX: n = 172 (51.2%), ECX + Bev: n = 164 (48.8%)), and 357 (48.5%) were classified as SARIFA-negative (ECX: n = 192 (53.8%), ECX + Bev: n = 165 (46.2%)). There was no significant difference in the frequency of SARIFA positivity or negativity between treatment arms in the ST03 trial (p = 0.458).
A single SARIFA-positive area was observed in a small proportion of cases. In the ST03 trial, 69 (21%) of 336 SARIFA-positive tumours had a single SARIFA-positive area although they had several tumour containing slides. Of these, one case had five tumour cells next to a fat cell and three cases showed a single tumour gland adjacent to a fat cell.
Relationship between SARIFA status and clinicopathologic characteristics
Clinicopathological characteristics at baseline for both trials stratified by SARIFA status and treatment arm, are provided in Tables 1 and 2.
In MAGIC ECF patients, tumours located in the stomach were more likely to be SARIFA-positive compared to tumours in the lower oesophagus or oesophagogastric junction (p = 0.004). Patients with SARIFA-positive tumours were more likely to have regional lymph node metastasis (p = 0.013). In MAGIC S patients, SARIFA positivity was associated with greater depth of invasion (pT) (p = 0.033). No relationship was seen between SARIFA status and other clinicopathological characteristics, see Table 1.
In the ST03 trial, SARIFA-positive tumours showed less primary tumour regression, were associated with higher ypT, presence of lymph node metastases, and positive resection margins in both treatment arms (see Table 2, all p < 0.001). SARIFA-positive tumours showed more often diffuse-type histology (ECX: p = 0.004; ECX + Bev: p < 0.001). No relationship was seen between the SARIFA status and other clinicopathological characteristics, see Table 2.
SARIFA status and body mass index
BMI data were available for all ST03 trial patients, but only available for 121 patients from the ECF arm in the MAGIC trial. SARIFA status was not associated with BMI in neither of the trials (ST03 p = 0.086, MAGIC p = 0.330). In both trials, there was no association of SARIFA status with WHO performance status (MAGIC: p = 0.472; ST03: p = 0.954).
Relationship between the SARIFA status and overall survival in the MAGIC trial patients
S patients with SARIFA-positive tumours had significantly shorter OS (hazard ratio (HR) 1.899; 95% confidence interval (CI) (1.285–2.806); plogrank = 0.001, Fig. 3a). The estimated median (95% CI) OS of S patients with SARIFA-positive tumours was 1.61 years (1.09–2.13 years) compared to 2.65 years (1.34–3.95 years) for S patients with SARIFA-negative tumours. 79% of S patients with SARIFA-positive tumours were dead at the end of the study period compared to 57% of the S patients with SARIFA-negative tumours.
a Kaplan Meier analysis of the MAGIC trial patients treated by surgery alone shows that patient with SARIFA-negative tumour have a significantly better survival (HR 1.899; 95 CI (1.285–2.806); plogrank = 0.001). b Kaplan Meier analysis of the MAGIC trial patients treated with peri-operative chemotherapy seems to suggest improved survival in patients with SARIFA-negative tumours. However, the difference is statistically not significant (HR 1.400; 95 CI (0.878–2.232); plogrank 0.155). c Kaplan Meier analysis comparing survival in the MAGIC trial patients with SARIFA-positive tumours between treatment arms shows a slightly improved survival after 2 years in the peri-operative chemotherapy arm. However, the difference is statistically not significant (HR 0.728; 95 CI (0.856–2.219); plogrank 0.190). d Kaplan Meier analysis comparing survival in the MAGIC trial patients with SARIFA-negative tumours between treatment arms shows no difference in survival (HR 0.985; 95 CI (0.697–1.478); plogrank 0.940).
ECF patients with SARIFA-negative tumours seem to have a better survival compared to ECF patients with SARIFA-positive tumours, however, the survival difference was not significant (HR 1.400; 95% CI (0.878–2.232), plogrank = 0.155, Fig. 3b). ECF patients with SARIFA-positive tumours had an estimated median (95% CI) OS of 1.65 years (0.95–2.36 years) compared to 2.64 years (1.40–3.89 years) in ECF patients with SARIFA-negative tumours. 30% of the ECF patients with SARIFA-positive tumours were alive at the end of the study period compared to 41% of the ECF patients with SARIFA-negative tumours. Survival of patients with SARIFA-positive tumours treated with perioperative chemotherapy (n = 37) seems to be slightly better after 2 years compared to patients with SARIFA-positive tumours treated with surgery only (n = 66), however, this difference was not significant (HR 0.728; 95 CI (0.856–2.219); plogrank 0.190; Fig. 3c). Patients with SARIFA-negative tumours had similar survival irrespective of treatment modality (HR 0.985; 95 CI (0.697–1.478); plogrank 0.940; Fig. 3d).
Relationship between the SARIFA status and overall survival in the ST03 trial patients
The initial exploration of the relationship between the SARIFA status and survival per treatment arm showed similar results in both treatment arms (see Supplementary Fig. S2). We therefore decided to analyse patients from both treatment arms together. ST03 patients with SARIFA-positive tumours had significantly shorter OS (HR 2.910; 95% CI (2.344–3.613); plogrank < 0.001, Fig. 4a). The estimated median (95% CI) OS of ST03 patients with SARIFA-positive tumours was 2.166 years (1.922–2.410 years) compared to 7.441 years (7.055- (median not reached) years) in patients with SARIFA-negative tumours. 64% of the patients with SARIFA-negative tumours were alive at the end of the study period compared to 30% of the patients with SARIFA-positive tumours.
a Kaplan Meier analysis of all ST03 trial patients shows that patients with SARIFA-negative tumours have a significantly better survival (HR 2.910; 95 CI (2.344–3.613); plogrank < 0.001). b Kaplan Meier analysis of all ST03 trial patients combining lymph node status (ypN) and SARIFA status shows that patients with SARIFA-positive ypN+ tumours have the poorest survival (HR 6.024, 95% CI 4.364–8.315), followed by SARIFA-negative ypN+ tumours (HR 2.354 95% CI 1.646–3.366), SARIFA-positive ypN0 tumours (HR 1.824 95% CI 1.146–2.904) and patients with SARIFA-negative ypN0 tumours have the best survival (plogrank < 0.001).
In multivariable OS analyses including the known prognostic factors ypT, ypN, ypR, and Mandard TRG in the model, the SARIFA status proved to be an independent prognostic factor (HR 1.974, 95% CI 1.555–2.507, p < 0.001, Supplementary Table S1).
Relationship between the SARIFA status, lymph node status and survival in the ST03 trial patients
Combining the SARIFA status and lymph node status (ypN0 vs ypN1 or higher (ypN+)) identified four groups of patients: SARIFA-negative ypN0 (n = 178, 26%), SARIFA-negative ypN+ (n = 177, 26%), SARIFA-positive ypN0 (n = 70, 10%) and SARIFA-positive ypN+ (n = 266, 38%). Patients with SARIFA-positive ypN+ tumours had the poorest survival (HR 6.024, 95% CI 4.364–8.315), followed by SARIFA-negative ypN+ tumours (HR 2.354 95% CI 1.646–3.366), SARIFA-positive ypN0 tumours (HR 1.824 95% CI 1.146–2.904) and patients with SARIFA-negative ypN0 tumours having the best survival (reference, p < 0.001, Fig. 4b).
Discussion
The introduction of perioperative or neoadjuvant combination chemotherapy has improved the outcome in patients with locally advanced resectable oesophagogastric cancers. Despite this progress, death due to locally recurrent disease or distant metastasis remains a major challenge in patients with oesophagogastric cancer [3]. In everyday clinical practice, the clinical decision on the postoperative treatment and surveillance strategy is highly relevant with regard to tolerability and quality of life. To address the urgent clinical need to improve stratification for risk of recurrent disease and/or prediction of OS after neoadjuvant therapy and surgical resection in order to personalise postoperative patient management, we evaluated the clinical utility of tumour-associated fat cells without desmoplastic stroma reaction at the invasion front (Stroma AReactive Invasion Front Areas (SARIFA)) in 985 oesophagogastric patients from the the MAGIC and the ST03 trial. Our own previous work showed that SARIFA status can be easily and reproducibly evaluated in routine Haematoxylin/Eosin (HE) stained tissue sections from resection specimens and suggested SARIFA status as new post-operative prognostic biomarker in oesophagogastric cancer patients. A summary of the current evidence including our own previous work regarding underlying mechanisms of the potential tumour promoting effect of tumour-associated adipocytes (CAAs) [12, 13] is presented in Fig. 1.
Our current study confirmed the prognostic value of the SARIFA status in surgery alone treated patients from the MAGIC trial. More importantly, this is the first study to suggest that the SARIFA status can also identify patient with a very poor prognosis when assessed in the post-chemotherapy resection specimens from the ST03 trial patients. Whilst we found a strong relationship between the SARIFA status, depth of tumour invasion, lymph node status and primary tumour regression grade, the SARIFA status nevertheless proved to be related to OS independently of known prognostic factors. In contrast to our findings in the ST03 trial, we only saw a non-significant trend of better survival of patients with SARIFA-negative tumours in the chemotherapy treated MAGIC trial patients. This might be related to the overall much lower number of patients with available SARIFA status in the MAGIC trial or could be related to differences in the sample collection of both trials. The tissue collection in the MAGIC trial was done retrospectively requesting a single representative block with highest tumour content per area, whereas tissue samples were collected prospectively in the ST03 trial requesting all slides from the resection specimen for central review. In particular in the post-chemotherapy resection specimen, a single tissue section selected to provide highest tumour content may not automatically include areas with adipocytes. This sampling bias could potentially also explain the lower frequency of SARIFA positivity in the chemotherapy treated patients in the MAGIC trial compared to the ST03 trial patients.
Lymph node status is known to be one of the most important prognostic factors in oesophagogastric cancer patients, more important than primary tumour regression as we showed in a previous study in the Oe02 and MAGIC trial patients. Combining the SARIFA status of the primary tumour with the lymph node status allowed us to identify subgroups of ypN0 patients with different survival. Whereas patients with SARIFA-negative ypN0 tumours had the best survival, the survival of patients with SARIFA-positive ypN0 tumours was significantly poorer and similar to patients with SARIFA-negative ypN+ tumours. This could suggest that the SARIFA status could be in particular clinically useful to identify ypN0 patients who may require adjuvant treatment which is different to the neoadjuvant regimen.
Lim et al. [14] showed that microvessel density increases in tumours where adipocytes are in contact with tumour cells which might be one of the underlying mechanisms for potential chemotherapy efficacy in SARIFA-positive cancers. Although the survival of SARIFA-positive patients was slightly better when treated with peri-operative chemotherapy compared to surgery alone in the MAGIC trial, this finding was not statistically significant and has to be interpreted with caution due to the small number of patients included in this subgroup analysis.
The main motivation for previous studies into tumour-associated fat cells was the observed association between obesity, cancer incidence, progression and therapy resistance. Results from our study show no association between BMI and SARIFA status. A higher BMI is partly associated with abdominal obesity, which is characterised by increased adipose tissue surrounding the intra-abdominal organs. High BMI does not necessarily mean that there is also more fatty tissue within the organ wall as such [15]. However, the supposed basic mechanism of an adipocyte-driven tumour progression in SARIFA-positive cancers may provide the basis for pharmacological intervention targeting the tumour cell metabolism with existing drugs such as Metformin, FABP4-inhibitors [16, 17] or CD36 [18, 19].
Our study has some limitations. This is a post-hoc analysis from a subset of patients from the the MAGIC and the ST03 trial. However, we have confirmed that the subsets are representative of the trial population who had a surgical resection (data not shown). The retrospective tissue collection of only a single tumour block from the MAGIC trial patients may have introduced bias. The SARIFA status can only be assessed in the resection specimen as fat cells are only present in the submucosa and deeper parts of the wall. As neoadjuvant chemotherapy is standard of care in patients with oesophagogastric cancer in the West, it would be clinically desirable to assess the predictive value of the SARIFA status in the pretreatment endoscopic biopsy in order to inform patient management. However, when screening pretreatment endoscopic biopsies from 100 randomly selected ST03 trial patients, only 4% of patients actually had few adipocytes present in the included submucosa, supporting our view that reliable evaluation of the SARIFA status in endoscopic biopsies is not feasible. Thus, further work is required to establish whether there are histological or molecular features measurable at the luminal (endoscopically reachable) tumour surface which are characteristics for the SARIFA status.
Conclusions
The results from the current study assessing the SARIFA status in resection specimens of oesophagogastric cancer patients after neoadjuvant chemotherapy from two phase III trials suggest that SARIFA status could be a clinically useful biomarker to identify patients with a high risk of recurrent disease after initial neoadjuvant chemotherapy who might benefit from a different therapeutic approach in the adjuvant setting. Evaluation of the SARIFA status can be done in routinely available patient material e.g. does not require extra material or procedures beyond routine histopathology and has a high interobserver agreement. Thus, this biomarker could be implemented into routine practice at relatively low costs and be reported within a clinically acceptable turnaround time. Further studies are warranted to assess the potential predictive value for already existing vasculature or lipid metabolism targeting agents.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl J Med. 2006;355:11–20.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101–15.
Kemi N, Eskuri M, Herva A, Leppanen J, Huhta H, Helminen O, et al. Tumour-stroma ratio and prognosis in gastric adenocarcinoma. Br J Cancer. 2018;119:435–9.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56.
Martin B, Grosser B, Kempkens L, Miller S, Bauer S, Dhillon C, et al. Stroma AReactive Invasion Front Areas (SARIFA)—a new easily to determine biomarker in colon cancer—results of a retrospective study. Cancers. 2021;13:4880.
Grosser B, Gluckstein MI, Dhillon C, Schiele S, Dintner S, VanSchoiack A, et al. Stroma AReactive Invasion Front Areas (SARIFA)—A new prognostic biomarker in gastric cancer related to tumor-promoting adipocytes. J Pathol. 2022;256:71–82.
Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357–70.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–6.
Duong MN, Geneste A, Fallone F, Li X, Dumontet C, Muller C. The fat and the bad: mature adipocytes, key actors in tumor progression and resistance. Oncotarget. 2017;8:57622–41.
Cui MY, Yi X, Zhu DX, Wu J. The role of lipid metabolism in gastric cancer. Front Oncol. 2022;12:916661.
Lim S, Hosaka K, Nakamura M, Cao Y. Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget. 2016;7:38282–91.
Shuster A, Patlas M, Pinthus J, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10.
Floresta G, Cilibrizzi A, Abbate V, Spampinato A, Zagni C, Rescifina A. FABP4 inhibitors 3D-QSAR model and isosteric replacement of BMS309403 datasets. Data Brief. 2019;22:471–83.
Huang Y, Jin C, Zheng Y, Li X, Zhang S, Zhang Y, et al. Knockdown of lncRNA MIR31HG inhibits adipocyte differentiation of human adipose-derived stem cells via histone modification of FABP4. Sci Rep. 2017;7:8080.
Pan J, Fan Z, Wang Z, Dai Q, Xiang Z, Yuan F, et al. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3beta/beta-catenin pathway. J Exp Clin Cancer Res. 2019;38:52.
Sp N, Kang DY, Kim DH, Park JH, Lee HG, Kim HJ, et al. Nobiletin inhibits CD36-dependent tumor angiogenesis, migration, invasion, and sphere formation through the Cd36/Stat3/NF-Kappa b signaling Axis. Nutrients. 2018;10:772.
McKillop IH, Girardi CA, Thompson KJ. Role of fatty acid binding proteins (FABPs) in cancer development and progression. Cell Signal. 2019;62:109336.
Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: from cancer progression to treatment. Prog Lipid Res. 2020;80:101055.
Guo Y, Wang ZW, Su WH, Chen J, Wang YL. Prognostic value and immune infiltrates of ABCA8 and FABP4 in stomach adenocarcinoma. Biomed Res Int. 2020;2020:4145164.
Correa LH, Correa R, Farinasso CM, de Sant’Ana Dourado LP, Magalhaes KG. Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: new implications in cancer progression. Front Immunol. 2017;8:1129.
Acknowledgements
HIG is supported in part by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Funding
Tissue collection/scanning was funded by Cancer Research UK (PI: HIG, grant ward C26441/A8944; C26441/A16251). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors revised the article critically, contributed to it with reflective improvements, and approved the final version. BM, HIG and BG contributed to the study’s conception and design. BM, HIG and BG contributed to the data acquisition process. Finally, BM, HIG, BG, JE, NGR, DC, MN, REL, WHA and MT contributed to the data analysis and interpretation.
Corresponding authors
Ethics declarations
Competing interests
DC has received grant funding from MedImmune, Clovis, Eli Lilly, 4SC, Bayer, Celgene, Leap and Roche not related to the current study. HIG Grabsch received honoraria from Astra Zeneca and Bristol Myers Squibb not related to the study. MT has received honoraria from Janssen, Roche and MedUpdate not related to the study.
Ethics approval and consent to participate
This study was approved by the UK NRES Committee London-South East (IRAS ID 144220). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grosser, B., Emmerson, J., Reitsam, N.G. et al. Stroma AReactive Invasion Front Areas (SARIFA) improves prognostic risk stratification of perioperative chemotherapy treated oesophagogastric cancer patients from the MAGIC and the ST03 trial. Br J Cancer 130, 457–466 (2024). https://doi.org/10.1038/s41416-023-02515-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02515-4