Abstract
Background
Anti-EGFR-based therapies have limited success in HNSCC patients. Predictive biomarkers are needed to identify the patients most likely to benefit from these therapies. Here, we present predictive and prognostic associations of different cancer stem cell markers in HPV-negative locally advanced (LA) HNSCC patients.
Methods
Pretreatment tumour tissues of 404 HPV-negative LA-HNSCCs patients, a subset of—phase 3-randomised study comparing cisplatin-radiation(CRT) and nimotuzumab plus cisplatin-radiation(NCRT) were examined. The expression levels of CD44, CD44v6, CD98hc, ALDH1A1, SOX2 and OCT4A were evaluated using immunohistochemistry. Progression-free survival(PFS), loco-regional control(LRC),- and overall survival(OS) were estimated by Kaplan–Meier method. Hazard ratios were estimated by Cox proportional hazard models.
Results
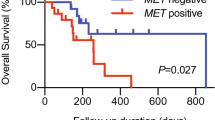
NCRT showed significantly improved OS with low membrane expression of CD44 compared to CRT [HR (95% CI) = 0.63 (0.46–0.88)]. Patients with low CD44v6 also showed better outcomes with NCRT [LRC: HR (95% CI) = 0.25 (0.10–0.62); OS: HR (95% CI) = 0.38 (0.19–0.74)]. No similar benefit with NCRT observed in patients with high CD44 or CD44v6 expression. Bootstrap resampling confirmed the predictive effect of CD44 (Interaction P = 0.015) and CD44v6 (Interaction P = 0.041) for OS. Multivariable Cox analysis revealed an independent negative prognostic role of CD98hc membrane expression for LRC [HR (95% CI) = 0.63(0.39–1.0)] and OS[HR (95% CI) = 0.62 (0.40–0.95)].
Conclusions
CD44 and CD44v6 are potential predictive biomarkers for NCRT response. CD98hc emerged as an independent negative prognostic biomarker.
Clinical trial registration
Registered with the Clinical Trial Registry of India (Trial registration identifier—CTRI/2014/09/004980).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this manuscript (and its supplementary information file). However, if required we can submit the clinical outcomes/follow-up data and biomarker data.
References
Braakhuis BJ, Brakenhoff RH, Leemans CR. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: biological risk factors. Ann Oncol. 2012;23(Suppl 10):x173–7.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. https://doi.org/10.1038/nrc2982
Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–83. https://doi.org/10.1038/s41571-019-0227-z
Tian Y, Lin J, Tian Y, Zhang G, Zeng X, Zheng R, et al. Efficacy and safety of anti-EGFR agents administered concurrently with standard therapies for patients with head and neck squamous cell carcinoma: a systematic review and meta-analysis of randomized controlled trials. Int J Cancer. 2018;142:2198–206. https://doi.org/10.1002/ijc.31157
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. https://doi.org/10.1016/S0140-6736(18)32779-X
Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. https://doi.org/10.1016/S0140-6736(18)32752-1
Byeon HK, Ku M, Yang J. Beyond EGFR inhibition: multilateral combat strategies to stop the progression of head and neck cancer. Exp Mol Med. 2019;51:1–14. https://doi.org/10.1038/s12276-018-0202-2
Chen LF, Cohen EE, Grandis JR. New strategies in head and neck cancer: understanding resistance to epidermal growth factor receptor inhibitors. Clin Cancer Res. 2010;16:2489–95. https://doi.org/10.1158/1078-0432.CCR-09-2318
Patel U, Pandey M, Kannan S, Samant TA, Gera P, Mittal N, et al. Prognostic and predictive significance of nuclear HIF1alpha expression in locally advanced HNSCC patients treated with chemoradiation with or without nimotuzumab. Br J Cancer. 2020. https://doi.org/10.1038/s41416-020-01064-4.
Bossi P, Resteghini C, Paielli N, Licitra L, Pilotti S, Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. 2016;7:74362–79. https://doi.org/10.18632/oncotarget.11413
Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol. 2013;8:173–81. https://doi.org/10.1007/s11523-013-0257-x
Pinto C, Barone CA, Girolomoni G, Russi EG, Merlano MC, Ferrari D, et al. Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist. 2011;16:228–38. https://doi.org/10.1634/theoncologist.2010-0298
Allan DG. Nimotuzumab: evidence of clinical benefit without rash. Oncologist. 2005;10:760–1. https://doi.org/10.1634/theoncologist.10-9-760
Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1:41–8. https://doi.org/10.4161/mabs.1.1.7509
Patil VM, Noronha V, Joshi A, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. 2019;125:3184–97. https://doi.org/10.1002/cncr.32179
Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–8. https://doi.org/10.1073/pnas.0610117104
Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. https://doi.org/10.1038/nrm1004
Fox SB, Fawcett J, Jackson DG, Collins I, Gatter KC, Harris AL, et al. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994;54:4539–46.
Ioachim E, Assimakopoulos D, Goussia AC, Peschos D, Skevas A, Agnantis NJ. Glycoprotein CD44 expression in benign, premalignant and malignant epithelial lesions of the larynx: an immunohistochemical study including correlation with Rb, p53, Ki-67 and PCNA. Histol Histopathol. 1999;14:1113–8. https://doi.org/10.14670/HH-14.1113
Senbanjo LT, Chellaiah MA. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18 https://doi.org/10.3389/fcell.2017.00018
Han S, Huang T, Li W, Wang X, Wu X, Liu S, et al. Prognostic value of CD44 and its isoforms in advanced cancer: a systematic meta-analysis with trial sequential analysis. Front Oncol. 2019;9:39 https://doi.org/10.3389/fonc.2019.00039
Martens-de Kemp SR, Brink A, Stigter-van Walsum M, Damen JM, Rustenburg F, Wu T, et al. CD98 marks a subpopulation of head and neck squamous cell carcinoma cells with stem cell properties. Stem Cell Res. 2013;10:477–88. https://doi.org/10.1016/j.scr.2013.02.004
Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci USA. 2005;102:355–60. https://doi.org/10.1073/pnas.0404852102
Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373–82. https://doi.org/10.1242/jcs.096040
Digomann D, Kurth I, Tyutyunnykova A, Chen O, Lock S, Gorodetska I, et al. The CD98 heavy chain is a marker and regulator of head and neck squamous cell carcinoma radiosensitivity. Clin Cancer Res. 2019;25:3152–63. https://doi.org/10.1158/1078-0432.CCR-18-2951
Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–13. https://doi.org/10.1016/j.bbrc.2009.05.048
Dong Y, Ochsenreither S, Cai C, Kaufmann AM, Albers AE, Qian X. Aldehyde dehydrogenase 1 isoenzyme expression as a marker of cancer stem cells correlates to histopathological features in head and neck cancer: a meta-analysis. PLoS ONE. 2017;12:e0187615 https://doi.org/10.1371/journal.pone.0187615
Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. https://doi.org/10.1016/j.stem.2012.12.007
Simandi Z, Horvath A, Wright LC, Cuaranta-Monroy I, De Luca I, Karolyi K, et al. OCT4 acts as an integrator of pluripotency and signal-induced differentiation. Mol Cell. 2016;63:647–61. https://doi.org/10.1016/j.molcel.2016.06.039
Dong Z, Liu G, Huang B, Sun J, Wu D. Prognostic significance of SOX2 in head and neck cancer: a meta-analysis. Int J Clin Exp Med. 2014;7:5010–20.
Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, et al. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94 https://doi.org/10.1186/1479-5876-8-94
Bayo P, Jou A, Stenzinger A, Shao C, Gross M, Jensen A, et al. Loss of SOX2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol Oncol. 2015;9:1704–19. https://doi.org/10.1016/j.molonc.2015.05.006
Keysar SB, Le PN, Miller B, Jackson BC, Eagles JR, Nieto C, et al. Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djw189.
Koo BS, Lee SH, Kim JM, Huang S, Kim SH, Rho YS, et al. Oct4 is a critical regulator of stemness in head and neck squamous carcinoma cells. Oncogene. 2015;34:2317–24. https://doi.org/10.1038/onc.2014.174
Bhosale PG, Pandey M, Desai RS, Patil A, Kane S, Prabhash K, et al. Low prevalence of transcriptionally active human papilloma virus in Indian patients with HNSCC and leukoplakia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:609–18. https://doi.org/10.1016/j.oooo.2016.06.006. e7
Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71.
Clark GM. Prognostic factors versus predictive factors: examples from a clinical trial of erlotinib. Mol Oncol. 2008;1:406–12. https://doi.org/10.1016/j.molonc.2007.12.001
Polley MY, Freidlin B, Korn EL, Conley BA, Abrams JS, McShane LM. Statistical and practical considerations for clinical evaluation of predictive biomarkers. J Natl Cancer Inst. 2013;105:1677–83. https://doi.org/10.1093/jnci/djt282
Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51:191–200. https://doi.org/10.1136/mp.51.4.191
Toyoda M, Kaira K, Shino M, Sakakura K, Takahashi K, Takayasu Y, et al. CD98 as a novel prognostic indicator for patients with stage III/IV hypopharyngeal squamous cell carcinoma. Head Neck. 2015;37:1569–74. https://doi.org/10.1002/hed.23797
Rietbergen MM, Martens-de Kemp SR, Bloemena E, Witte BI, Brink A, Baatenburg de Jong RJ, et al. Cancer stem cell enrichment marker CD98: a prognostic factor for survival in patients with human papillomavirus-positive oropharyngeal cancer. Eur J Cancer. 2014;50:765–73. https://doi.org/10.1016/j.ejca.2013.11.010
Linge A, Lock S, Gudziol V, Nowak A, Lohaus F, von Neubeck C, et al. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clin Cancer Res. 2016;22:2639–49. https://doi.org/10.1158/1078-0432.CCR-15-1990
Perez A, Neskey DM, Wen J, Pereira L, Reategui EP, Goodwin WJ, et al. CD44 interacts with EGFR and promotes head and neck squamous cell carcinoma initiation and progression. Oral Oncol. 2013;49:306–13. https://doi.org/10.1016/j.oraloncology.2012.11.009
Morath I, Jung C, Leveque R, Linfeng C, Toillon RA, Warth A, et al. Differential recruitment of CD44 isoforms by ErbB ligands reveals an involvement of CD44 in breast cancer. Oncogene. 2018;37:1472–84. https://doi.org/10.1038/s41388-017-0030-1
Kim Y, Lee YS, Choe J, Lee H, Kim YM, Jeoung D. CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem. 2008;283:22513–28. https://doi.org/10.1074/jbc.M708319200
Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–8. https://doi.org/10.1001/archotol.132.7.771
Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–40. https://doi.org/10.1074/jbc.M507734200
Krishnamachary B, Penet MF, Nimmagadda S, Mironchik Y, Raman V, Solaiyappan M, et al. Hypoxia regulates CD44 and its variant isoforms through HIF-1alpha in triple negative breast cancer. PLoS ONE. 2012;7:e44078 https://doi.org/10.1371/journal.pone.0044078
Liang G, Li S, Du W, Ke Q, Cai J, Yang J. Hypoxia regulates CD44 expression via hypoxia-inducible factor-1alpha in human gastric cancer cells. Oncol Lett. 2017;13:967–72. https://doi.org/10.3892/ol.2016.5473
Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826:272–96. https://doi.org/10.1016/j.bbcan.2012.04.008
Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–52. https://doi.org/10.1158/0008-5472.CAN-10-3320
Baumeister P, Hollmann A, Kitz J, Afthonidou A, Simon F, Shakhtour J, et al. High expression of EpCAM and Sox2 is a positive prognosticator of clinical outcome for head and neck carcinoma. Sci Rep. 2018;8:14582 https://doi.org/10.1038/s41598-018-32178-8
Chung JH, Jung HR, Jung AR, Lee YC, Kong M, Lee JS, et al. SOX2 activation predicts prognosis in patients with head and neck squamous cell carcinoma. Sci Rep. 2018;8:1677 https://doi.org/10.1038/s41598-018-20086-w
Pedregal-Mallo D, Hermida-Prado F, Granda-Diaz R, Montoro-Jimenez I, Allonca E, Pozo-Agundo E, et al. Prognostic significance of the pluripotency factors NANOG, SOX2, and OCT4 in head and neck squamous cell carcinomas. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12071794.
Schrock A, Bode M, Goke FJ, Bareiss PM, Schairer R, Wang H, et al. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis. 2014;35:1636–42. https://doi.org/10.1093/carcin/bgu094
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–35. https://doi.org/10.1093/jnci/86.11.829
Acknowledgements
We acknowledge all patients and investigators who contributed to the study. Additionally, we would like to thank Cactus Communications Pvt. Ltd., Mumbai, India for their assistance in English language editing.
Funding
Council of Scientific & Industrial Research, fellowship to Usha Patel, Grant/Award Number: 09/513(0099)/2014‐EMR‐I; Science and Engineering Research Board, Grant/Award Number: EMR/2015/001591; Tata Memorial Centre, Seed In Air grant, Grant/Award Number: TMC/SIA/2696.
Author information
Authors and Affiliations
Contributions
Methodology: UP, SM, VS and MBM; scoring of IHC slides: SR, NM, PG and AP; data curation and formal analysis: UP and SK; project administration: UP and MBM; writing—original draft: UP and MBM; writing—review and editing: UP, MBM, SR, NM and SK; conducting the trial: AJ, VN, VMP and KP; conceptualisation and supervision: MBM; funding acquisition and resources: MBM. All authors approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Tata Memorial Center (IEC approval 50 of 2011) and was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Consent for publication
NA
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Patel, U., Kannan, S., Rane, S.U. et al. Prognostic and predictive roles of cancer stem cell markers in head and neck squamous cell carcinoma patients receiving chemoradiotherapy with or without nimotuzumab. Br J Cancer 126, 1439–1449 (2022). https://doi.org/10.1038/s41416-022-01730-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01730-9
This article is cited by
-
Population-Based Prognostic Models for Head and Neck Cancers Using National Cancer Registry Data from Taiwan
Journal of Epidemiology and Global Health (2024)
-
A state-of-the-art review on the NRF2 in Hepatitis virus-associated liver cancer
Cell Communication and Signaling (2023)