Abstract
Bruxism may lead to changes or damage to the oral and perioral tissues. Bruxism may occur during sleep or when awake. Many patients will not require active management; however, for some, intervention is required. Control of bruxism may be difficult, if not impossible, but the need exists for preservation of the dentition and quality of life. A prediction of risk to the tissues for the planning of interventions is difficult and relies upon evidence of past damage and assessment of future risks. Treatment options may need to be imaginative and rescuable. This series of papers will review the aetiology of bruxism, its impacts and treatment strategies for persistent bruxers who are at risk of, or suffering, tissue damage.
Key points
-
Bruxism is not just simply grinding of the teeth and is more than temporomandibular disorder.
-
Bruxism is a centrally driven sleep disorder that often has a familial and long patient history.
-
Bruxism is initiated and/or exacerbated by selective serotonin reuptake inhibitors.
-
Bruxism can be very destructive to the dental and oral tissues and leads to quality of life issues. Decisions as to when to intervene are difficult and an intervention needs assessment is introduced.
Similar content being viewed by others
Introduction
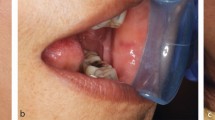
Bruxism was defined in 2013 as 'repetitive jaw-muscle activity characterised by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible' by international concensus,1 with supplemental classification of sleep or awake bruxism. It has also been classified as a sleep movement disorder.2,3 Tooth surface loss (TSL) is a well-recognised phenomenon, evident in all age groups, with well-defined management strategies.4,5,6,7 TSL is multifactorial in nature but one aspect leading to TSL is parafunctional behaviours of the oral and perioral tissues, in particular, bruxism. Damage from bruxism can be minor or substantial, affecting hard tissues, with attrition of occlusal surfaces of the teeth, fractures of teeth or restorations (Fig. 1), including implant retained restorations (Fig. 2) and can lead to damage or changes in the soft tissues via soft tissue trauma (Fig. 3), ulceration, hypertrophy or hyperplasia (Fig. 4). There have even been reports of parotid duct obstruction with consequent symptoms.8,9 Function may be impacted and aesthetics changed, leading to negative psychological impact and in some, social isolation and poor quality of life.10,11,12 Persistent increased muscular activity may become symptomatic, with facial pain and aspects of temporomandibular symptoms, but also may be symptomless. The impact of the activity can damage tissues that may then become painful, for example with a fracture of a tooth, but often with no apparent pre-existing history of bruxism. Control of bruxism and other parafunctional activity and the damage that these can produce may significantly improve function and quality of life, but in addition, may also improve outcomes for patients with reduced clinical time and reduced financial burden, to both the patient and the health service. A primary issue with bruxists is predicting the long-term outcomes. While many will not require active intervention, others do require methods of protection of the dentition and tissues. Risk assessments for prescribing are difficult and rely on historical evidence of tissue damage and ongoing behaviour patterns, with real-time predictors of future risks.
Epidemiology
While increased muscular activity may often be symptom free, it is common. Incidence of bruxism is reported as between 5-30%,13,14 with prevalence appearing similar across the world. Bruxing may occur at night (sleep bruxism), during the day (awake bruxism) or both. There is no sex predilection, but incidence decreases with age, with the peak incidence lying in adolescence and younger adulthood, with a slow and linear decline13 in incidence over 40 years of age to a rate of about 3% in older patients (greater than 60 years).2,13,14 There is an association, although limited, with stress, anxiety and poor mental health and a number of other environmental, extrinsic factors.
Is it bruxism?
Or when is bruxism not bruxism? 'Bruxism' can be a catchall term used by many to describe grinding or clenching of the teeth, primarily at night. Despite the above definition, it is important to draw a distinction between 'just' increased functional activity and 'real' bruxism. Rhythmic masticatory muscle activity (RMMA) occurs during sleep but appears to be a normal component of sleep behaviour activity for many. Much of the literature looks at sleep bruxism and there is little considering awake bruxism. Lavigne and colleagues15 studied both (sleep) bruxers and non-bruxers, finding that the non-bruxist patients also demonstrated this RMMA during sleep, but, as might be expected, there were differences - the bruxing patients showed more episodes of RMMA, with increased bursts per episode and bursts of a greater amplitude yet at a shorter duration. This may be an important finding, as it would appear that bruxing can substantially increase the force applied to teeth.16,17,18 It seems reasonable to conclude that the cumulative force applied to the teeth is the difference between the two similar activities and leads to the potentially destructive impact of sleep bruxism. Others1,19 have suggested that in excess of four episodes of increased muscle activity and grinding per hour is a breakeven point above which damage can occur to tissues. The difficulty is identifying one from the other and predicting how sleep or awake bruxism will impact in the long-term. This distinction appears repeatedly through the literature but with little clarity in deciding on the defining characteristics of (destructive) bruxism from RMMA, despite the real need for this to aid planning and avoid unnecessary damage to tissues and also to avoid unnecessary treatment. In an expansion of the definition paper, Manfredini and co-authors20 explore the concept of bruxism as a continuity from 'simple' nocturnal muscular activity to destructive bruxism, acknowledging the potential complexity of determining where a patient may lie on the spectrum. The title of the paper tells it all: 'From cut-off points to a continuum spectrum'. The concept that it is possible to identify a limit beyond which bruxism is destructive is probably inherently flawed, as it is different for every patient.
It is also important to draw a distinction between temporomandibular disorders (TMDs) and bruxism. TMDs are best considered as a biopsychosocial phenomenon, with patients responding to a range of factors initiating and maintaining parafunctional activity, with consequent symptomology of pain or joint issues. Painful TMDs are not the same as bruxism, nor RMMA; TMDs are typically responsive to environmental factors changing behaviour and there is little solid evidence that pain in TMDs is linked with 'true' bruxism (awake or sleep). While there would appear to be a number of genetic risk factors for painful TMD, the genetic factors are broadly different to those for sleep bruxism.21
Diagnosis of bruxism is typically completed via questionnaire, symptom history and examination, with quantifying assessments of bruxism done by the use of electromyography (EMG) and polysomnography (PSG)1. In reality, these do not actually tell the clinician if the patient is bruxing, only that the muscles are operating when not expected at night and that the teeth may be in contact. The activity pattern may infer bruxism, for example, the international research criteria for (sleep) bruxism1,20 is the following:
-
Four or more episodes of increased activity per hour
-
More than 6 bursts of activity per episode and/or 25 activity bursts per hour of sleep
-
At least two episodes of grinding sound in a research environment.
There is, however, a lack of validity of these definitions as there is no actual solid evidence that these are correct. A method of recording intercuspal pressure might assist in the more accurate determination of what is, or is not, bruxism. However, the reality is that the use of EMG, PSG and bite force measures is not practical outside the sleep laboratory. As the ability of the researcher and clinician to gauge a specific point when bruxism becomes a 'problem' is so vague, the clinician will inevitably need to rely upon careful history and comprehensive examination, with real time monitoring and understanding of the behaviour of the tissues, coupled with the astute application of clinical judgement.
It is interesting to note that some papers consider generic bruxism as a risk factor for other issues and tissue damage. That also means that patients who brux do not necessarily produce tissue damage or tooth wear, as outlined above. For this paper series, the authors will consider bruxism, whether sleep or awake, as a given and that it is leading to tissue damage. Despite the assertion above that identifying a cut-off point for determining when bruxism is destructive is probably not possible, the authors will consider the 'when you really need to intervene' and the 'how to intervene', in what they hope is a rational format.
Aetiology
The aetiology of bruxism is considered a multifactorial, but centrally driven, process. This will be reviewed under aspects that can predispose, initiate and promote and those that maintain bruxing (Table 1).
Predisposing factors
Predisposing factors are risk factors that may make a patient more likely to initiate bruxing activity. These may be intrinsic or extrinsic.
Intrinsic factors
Discussions with patients frequently (but not exclusively) reveal a long-standing history of teeth clenching or grinding. A history of childhood grinding at night is common and a familial history is also frequent. These histories fit with assessment of bruxism as a sleep movement disorder. Research into potential genetic drivers to bruxism suggests that there are both genetic and epigenetic aspects leading to an individual who is at risk of developing bruxism, in particular, sleep bruxism22 and that bruxism is likely to be centrally driven rather than a result of peripheral influences, such as from the occlusion. Research has shown that several genes relating to dopamine biology appear to be associated with bruxism in children.23,24,25 Age leads to reduced dopamine receptors in the brain, which would align with reduced bruxism with age.
Also, 5-hydroxytryptamine (serotonin) metabolism appears to play a role in the central drive24,26,27 and selective 5-hydroxytryptamine reuptake inhibitors have been identified as being implicated in bruxism28,29 in adults.
Bruxism may also present as a facet of other nervous system disorders, such as Parkinsonian disorders, cerebral palsy, or other movement disorders.30
Sleep disorders may pose an intrinsic risk for bruxism. The majority of sleep bruxism episodes appear to occur during non-rapid eye movement and light sleep at periods of microarousal from sleep; bruxing appears in episodes around the period of arousal. Such episodes are frequent (8-15 times an hour) but last only a short time (3-10 seconds).2 Obstructive sleep apnoea may seem a logical culprit, but the association with bruxing is limited and in fact, the association with snoring is greater.31 It is proposed that sleep bruxism has a protective effect by moving the mandible when hypopnoea occurs, which would be a centrally driven arousal process.2,32 It is also postulated that bruxing activity can produce lubrication of the oropharyngeal tissues by inducing salivary flow and is associated with swallowing activity in a substantial proportion of cases.33
Extrinsic factors
The primary extrinsic risk factor predisposing to bruxism appears to be psychological - stress, anxiety and depression. This appears to be more strongly associated with daytime awake bruxism than sleep bruxism, which would make sense given the likely genetic and central nature of sleep bruxism. Literature2,34,35,36,37,38,39 on this, however, is mixed and the associations not completely clear. One confounding factor in this association is the need for antidepressant therapy for patients with mental health issues, as some of these agents appear to be linked with bruxism. This is explored below. Other predisposing factors include smoking (which links with obstructive sleep apnoea), medication and recreational drug use. Alcohol use appears to have relatively limited association.31
Initiating factors
In most cases, it is difficult to pin down a specific initiation, especially when there is a long history of bruxism; however, there is a range of risks that may lead to initiation of bruxism. Perhaps primary among these are mental health issues. There is a clear association between mental health issues and awake bruxism,34,35,36,37,38,39 with a significant increased risk of awake bruxism occurring during episodes of stress or anxiety. The relationship with sleep bruxism is less clear cut. Mental health disorders such as schizophrenia are also implicated40,41 but those such as the personality disorders seem less commonly associated. Eating disorders do not seem to specifically increase the incidence of bruxism but are rarely mentioned in bruxism literature.
There is increasingly good evidence of a link with selective 5-hydroxytryptamine reuptake inhibitor (selective serotonin reuptake inhibitor [SSRI]) use,42 especially citalopram and sertraline, which may be dose linked, as well as selective 5-hydroxytryptamine and noradrenaline reuptake inhibitors (serotonin and norepinephrine reuptake inhibitors [SNRIs]) such as venlafaxine, which appears strongly linked with bruxism. Kara and colleagues43 demonstrated the onset or exacerbation of sleep bruxism in patients commencing SSRI therapy. The onset appears fairly rapid - within days. Here again, the mechanism appears to be related to dopamine activity and its disturbance by SSRIs. Dopamine inhibits spontaneous masticatory muscle activity and 5-hydroxytryptamine interferes with this action. Loss of this inhibition may therefore disinhibit muscular control and lead to the initiation of bruxism.44 While their study looked at sleep bruxism specifically, it is impossible to escape the view that this may operate in awake bruxism as well.
However, in general, the potential for changed function is linked to a change in muscle tone as a response to mental distress. As far back as the late 1980s, Rugh45 (quoted by Oekson)46 demonstrated increased muscle activity linked to stress, with clear linkage between stressful life episodes followed by sharp increases in muscular activity. Parallel evidence shows that mental health issues significantly increase the risk of symptoms of TMDs.47 Patients presenting with TMD largely show increased activity in the muscles of mastication, with bruxism frequently blamed for the development of painful TMD. Bruxism may be a risk factor for TMD48 but is not necessarily the cause of painful TMD. However, overall, it seems likely that the primary initiating factor for a proportion of cases (mainly awake bruxism) lies within the psychological axis of the condition.
In a few unusual cases, a trigger can be identified, such as trauma (for example, a car accident and associated whiplash). Neuroimaging has identified the involvement of parts of the brain associated with the hypothalamic-pituitary-adrenal axis (HPA) in bruxism and also implicated in TMD and post-traumatic stress disorder (PTSD).49,50 Disturbances in the HPA are significantly implicated in anxiety. Here, it is suggested that bruxism, PTSD, obsessive compulsive disorder and other stress-related psychiatric disorders are due to a dysfunction of this system and it is suggested that a negative feedback loop exists which may be important in the initiation or regulation of bruxing.49,51
A number of studies have shown a strong association between gastro-oesophageal reflux disorder (GORD)31,52,53 and bruxism. In experimental subjects without GORD, acidification of the stomach initiates bruxism. At first sight, this might seem an odd association and a direct cause and effect is not clear. However, it is proposed that the link may be related to microarousal episodes of sleep disturbance that then initiate bruxism, possibly to lubricate the oropharynx. Disturbed sleep is a common facet of GORD. There are confounding factors, such as the effects of stress on both bruxism and GORD; however, the work by Ohmure and colleagues54 clearly shows the initiation of bruxism in non-bruxists by acidification of the stomach. GORD is also an intrinsic risk factor for tooth surface loss that may then be accelerated by bruxism. The dental implications of GORD and acidification of the oral environment are well documented. Figure 5 shows a case of tooth surface loss in a very young bruxist (21 years old) with gastric reflux and significant mental health issues. There is obvious erosion of the teeth but there is also clear attrition with wear facets across cusps of the molars (Fig. 6). This patient would warrant early intervention to control tissue damage.
The evidence therefore suggests that bruxism is a centrally mediated phenomenon that has multiple possible routes and mechanisms for initiation.
Promoting and maintaining factors
There is relatively little literature looking at reasons that bruxing continues or is exacerbated. However, some aspects of the predisposing and initiating factors will also come into play.
Does bruxing actually matter? The impact of bruxism
Opinions on the importance or impact of bruxism vary. In their paper in 2018, Beddis et al.55 suggested that sleep bruxism did not require intervention in most cases but acknowledged that some patients will require intervention due to tissue damage. Yet, as noted, bruxism can lead to a range of issues that can impact on quality of life. In assessing need, it is important to consider the patient's presenting complaint and the historical evidence of damage from bruxism. However, it is clear that simple assessment of tooth wear (as opposed to tooth surface loss) per se, is a poor indicator of risk to the patient from bruxism of any origin.
When considering the impact of bruxism, whether awake or asleep, both hard and soft tissues should be considered. While extreme attrition of the dentition is very clear, changes within soft tissues may be more subtle. However, most changes are relatively easy to identify and flag during routine examination and a detailed examination may then be instigated to assess the risk posed to the patient's oral health. Issues to review are shown in Table 2.
The psychological impact varies tremendously. Some patients are remarkably sanguine and accept dramatic tooth (tissue) loss over time as just part of life, while others worry to an obsessive amount about the most minor loss of enamel. Discussions with patients who have significant worries in the presence of relatively minor changes often reveals an increased level of general (or trait) anxiety, mental health issues in general and stressed personal circumstances. The impact on cosmetics and the patient interaction with friends and family can be a very strong driver to seek correction of tooth surface loss, even when such correction is not warranted. In very crude stratification, older patients tend to be pragmatic, while younger patients tend to have a very low threshold for concern. This is an issue, as these significant levels of anxiety impact on the patient's quality of life and may lead to inappropriate demands for treatment.
Risk assessment is complex, involves a large amount of clinical judgement and can be fraught with potential pitfalls. A clear diagnosis of bruxism is required and this is well covered by Beddis et al.,55 although this looked specifically at sleep bruxism. The criteria for a diagnosis of sleep bruxism is noted previously and relies upon self-reported information with clinical signs, but the gold standard is polysomnography. This does, however, only address the sleep phase of bruxism and is not realistic for day-to-day dental practice. Diagnosis of awake bruxism must rely much more heavily on patient reports, behavioural analysis (for example, habits and daytime activities) and clinical signs.
Treatment need is driven by both the extent of tissue damage, the patient expectations and the modifying factors. A potential need stratification matrix is shown in Table 3, which may offer a method to enhance and rationalise the decision-making process, increasing decision defensibility. This assessment matrix has been trialled by the authors, both prospectively and retrospectively and appears to provide an accurate support to the assessment process. The clinical assessment allows completion of the matrix, with an overall assessment of low, medium, or high potential need for intervention.
Why an intervention needs assessment?
The issue facing patients is the potential of delays in intervention, until such time as the dentition is extensively damaged, requiring extensive reconstruction and with an impaired long-term prognosis for the dentition. While extensive damage is easy to identify, early identification of patients who are at significant risk of extensive tooth wear is less clear, yet more crucial for prevention of damage. The matrix is described as an intervention needs matrix, rather than a risk assessment matrix, as by definition, all patients in this group are risk patients.
Designing the intervention needs matrix
Design of a matrix requires assumptions and review to refine. Some aspects may seem speculative but have a logical design rationale. For example, the number of fractured teeth; three as a watershed might appear to be simple guesswork. However, it should be considered that the fracture of 3 (posterior) teeth represents approximately 20% of the functional posterior units - more if orthodontics has led to early premolar loss. Anterior teeth appear to show substantial fracture less commonly and small amounts of enamel chipping are not included as there are other confounding reasons for incisal edge fracture, not least external trauma. Multiple crowns of posterior teeth should be assumed to be likely to represent at least one fracture (more if confirmed with patient records or clear history) but may be ignored in patients presenting with a high caries rate as the caries is more likely to be the cause of extensive damage.
Soft tissue changes relate to muscular hypertrophy and other significant changes, but not those such as linea alba or tongue scalloping, as these present in many patients with non-destructive habits and 'simple' TMDs.
Each area is assigned a score, depending on the significance of the issue identified. For example, patients over 40 score low, as tooth wear will be more in line with age. A score of 13-17 equates to a low potential need, 18-21 as medium and greater than 22 as high. The scoring will not allow a score below 13 as this loses sensitivity; there is a need for dilution of some findings to avoid a situation where everyone scores in the 'treatment needed' group.
This material can then form the basis for discussions with patients as to the appropriateness of intervention (not the type of intervention). What these assessments cannot establish is a cut-off point beyond which treatment is positively indicated, nor when it is positively contra-indicated, which is in line with the earlier discussion in relation to the difference between cut-off points and the continuum of bruxism. In reality, this matrix looks at 'if' the patient will need intervention, rather than 'when'. Figures 7 and 8 show a 46-year-old patient with RMMA scoring 14 and when compared to Figures 5 and 6 (score 23), shows this variation of need in action.
Application of this matrix will be discussed in context in the next papers of the series.
Conclusion
Bruxism is much more than simply grinding of the teeth. Sleep bruxism is a different condition to awake bruxism, with different and multifactorial causation and risk factors. Sleep bruxism appears to be a sleep disorder, whereas awake bruxism is probably more associated with psychological stress and medication. In both cases, the clinician needs to deploy diagnostic skills with discretion and intelligence and must be aware of the potential damage that bruxism can cause to the oral tissues.
References
Lobbezoo F, Ahlberg J, Glaros A G et al. Bruxism defined and graded: an international consensus. J Oral Rehabil 2013; 40: 2-4.
Lavine G J, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil 2008; 35: 476-494.
American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Illinois: American Academy of Sleep Medicine, 2014.
Mehta S B, Banerji S, Millar B J, Suarez-Feito J-M. Current concepts on the management of tooth wear: part 1. Assessment, treatment planning and strategies for the prevention and the passive management of tooth wear. Br Dent J 2012; 212: 17-27.
Mehta S B, Banerji S, Millar B J, Suarez-Feito J-M. Current concepts on the management of tooth wear: part 2. Active restorative care 1: the management of localised tooth wear. Br Dent J 2012; 212: 73-82.
Mehta S B, Banerji S, Millar B J, Suarez-Feito J-M. Current concepts on the management of tooth wear: part 3. Active restorative care 2: the management of generalised tooth wear. Br Dent J 2012; 212: 121-127.
Mehta S B, Banerji S, Millar B J, Suarez-Feito J-M. Current concepts on the management of tooth wear: part 4. An overview of the restorative techniques and dental materials commonly applied for the management of tooth wear. Br Dent J 2012; 212: 169-177.
Teymoortash A, Hoch S, Weber D, Wilhelm T, Günzel T. Bruxism-induced parotitis: A retrospective case series analysis. Cranio 2020; 38: 115-121.
Capaccio P, Gaffuri M, Pignataro L, Assandri F, Pereira P, Farronato G. Recurrent parotid swelling secondary to masseter muscle hypertrophy: a multidisciplinary diagnostic and therapeutic approach. Cranio 2016; 34: 388-394.
Li M H M, Bernabé E. Tooth wear and quality of life among adults in the United Kingdom. J Dent 2016; 55: 48-53.
Sterenborg B A M M, Bronkhorst E M, Wetselaar P, Lobbezoo F, Loomans B A C, Huysmans M-C D N J M. The influence of management of tooth wear on oral health-related quality of life. Clin Oral Investig 2018; 22: 2567-2573.
Papagianni C E, van der Meulen M J, Naeije M, Lobbezoo F. Oral health-related quality of life in patients with tooth wear. J Oral Rehabil 2013; 40: 185-190.
Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain 2013; 27: 99-110.
Maluly M, Andersen M L, Dal-Fabbro C et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res 2013; DOI: 10.1177/0022034513484328.
Lavigne G J, Rompré P H, Poirier G, Huard H, Kato T, Montplaisir J Y. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res 2001; 80: 443-448.
Custodio W, Gomes S G F, Faot F, Garcia R C M R, Del Bel Cury A A. Occlusal force, electromyographic activity of masticatory muscles and mandibular flexure of subjects with different facial types. J Appl Oral Sci 2011; 19: 343-349.
Waltimo A, Nyström M, Könönen M. Bite force and dentofacial morphology in men with severe dental attrition. Scand J Dent Res 1994; 102: 92-96.
Gibbs C H, Mahan P E, Mauderli A, Lundeen H C, Walsh E K. Limits of human bite strength. J Prosthet Dent 1986; 56: 226-229.
Lavigne G J, Rompré P H, Montplaisir J Y. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res 1996; 75: 546-552.
Manfredini D, Ahlberg J, Wetselaar P, Svensson P, Lobbezoo F. The bruxism construct: From cut-off points to a continuum spectrum. J Oral Rehabil 2019; 46: 991-997.
Smith S B, Maixner D W, Greenspan J D et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain 2011; DOI: 10.1016/j.jpain.2011.08.005.
Lobbezoo F, Visscher C M, Ahlberg J, Manfredini D. Bruxism and genetics: a review of the literature. J Oral Rehabil 2014; 41: 709-714.
Tükel R, Gürvit H, Öztürk N et al. COMT Val158Met polymorphism and executive functions in obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2013; 25: 214-221.
Oporto 5th G H, Bornhardt T, Iturriaga V, Salazar L A. Single nucleotide polymorphisms in genes of dopaminergic pathways are associated with bruxism. Clin Oral Investig 2018; 22: 331-337.
Scariot R, Brunet L, Olsson B et al. Single nucleotide polymorphisms in dopamine receptor D2 are associated with bruxism and its circadian phenotypes in children. Cranio 2022; 40: 152-159.
Abe Y, Suganuma T, Ishii M et al. Association of genetic, psychological and behavioural factors with sleep bruxism in a Japanese population. J Sleep Res 2012; 21: 289-296.
Oporto 5th G H, Bornhardt T, Iturriaga V, Salazar L A. Genetic polymorphisms in the serotonergic system are associated with circadian manifestations of bruxism. J Oral Rehabil 2016; 43: 805-812.
Uca A U, Uğuz F, Kozak H H et al. Antidepressant-Induced Sleep Bruxism: Prevalence, Incidence, and Related Factors. Clin Neuropharmacol 2015; 38: 227-230.
Garrett A R, Hawley J S. SSRI-associated bruxism: A systematic review of published case reports. Neurol Clin Pract 2018; 8: 135-141.
Ella B, Ghorayeb I, Burbaud P, Guehl D. Bruxism in Movement Disorders: A Comprehensive Review. J Prosthodont 2017; 26: 599-605.
Castroflorio T, Bargellini A, Rossini G, Cugliari G, Deregibus A. Sleep bruxism and related risk factors in adults: A systematic literature review. Arch Oral Biol 2017; 83: 25-32.
Khoury S, Rouleau G A, Rompré P H, Mayer P, Montplaisir J Y, Lavigne G L. A significant increase in breathing amplitude precedes sleep bruxism. Chest 2008; 134: 332-337.
Miyawaki S, Lavigne G J, Pierre M, Guitard F, Montplaisir Y, Kato T. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep 2003; 26: 461-465.
Smardz J, Martynowicz H, Wojakowska A, Michalek-Zrabkowska M, Mazur G, Wieckiewicz M. Correlation between Sleep Bruxism, Stress, and Depression-A Polysomnographic Study. J Clin Med 2019; DOI: 10.3390/jcm8091344.
Przystańska A, Jasielska A, Ziarko M et al. Psychosocial Predictors of Bruxism. Biomed Res Int 2019; DOI: 10.1155/2019/2069716.
Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain 2009; 23: 153-166.
Gungormus Z, Erciyas K. Evaluation of the relationship between anxiety and depression and bruxism. J Int Med Res 2009; 37: 547-550.
Polmann H, Domingos F L, Melo G et al. Association between sleep bruxism and anxiety symptoms in adults: A systematic review. J Oral Rehabil 2019; 46: 482-491.
Tavares L M F, da Silva Parente Macedo L C, Duarte C M R, de Goffredo Filho G S, de Souza Tesch R. Cross-sectional study of anxiety symptoms and self-report of awake and sleep bruxism in female TMD patients. Cranio 2016; 34: 378-381.
Winocur E, Hermesh H, Littner D, Shiloh R, Peleg L, Eli I. Signs of bruxism and temporomandibular disorders among psychiatric patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 60-63.
Gurbuz O, Alatas G, Kurt E. Prevalence of temporomandibular disorder signs in patients with schizophrenia. J Oral Rehabil 2009; 36: 864-871.
Garrett A R, Hawley J S. SSRI-associated bruxism. A systematic review of published case reports. Neurol Clin Pract 2018; 8: 135-141.
Kara I M, Ertaş E T, Ozen E et al. BiteStrip Analysis of the Effect of Fluoxetine and Paroxetine on Sleep Bruxism. Arch Oral Biol 2017; 80: 69-74.
Oulis P, Dimitrakopoulos S, Konstantakopoulos G, Tsaltas E, Kollias K. Low-dose aripiprazole in the treatment of SSRI-induced bruxism. J Neuropsychiatry Clin Neurosci 2012; DOI: 10.1176/appi.neuropsych.11070175.
Rugh J D, Harlan J. Nocturnal bruxism and temporomandibular disorders. Adv Neurol 1988; 49: 329-341.
Oekson J. Management of Temporomandibular Disorders and Occlusion. 4th ed. Florida: Mosby, 1997.
Fillingim R B, Ohrbach R, Greenspan J D et al. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 2013; DOI: 10.1016/j.jpain.2013.06.009.
Sierwald I, John M T, Schierz O et al. Association of temporomandibular disorder pain with awake and sleep bruxism in adults. J Orofac Orthop 2015; 76: 305-317.
Dharmadhikari S, Romito L M, Dzemidzic M et al. GABA and glutamate levels in occlusal splint-wearing males with possible bruxism. Arch Oral Biol 2015; 60: 1021-1029.
Etkin A, Wager T D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476-1488.
Fan X, Qu F, Wang J-J, Du X, Liu W-C. Decreased γ-aminobutyric acid levels in the brainstem in patients with possible sleep bruxism: A pilot study. J Oral Rehabil 2017; 44: 934-940.
Mengatto C M, da Silveira Dalberto C, Scheeren B, de Barros S G S. Association between sleep bruxism and gastroesophageal reflux disease. J Prosthet Dent 2013; 110: 349-355.
Li Y, Yu F, Niu L, Long Y, Tay F R, Chen J. Association between bruxism and symptomatic gastroesophageal reflux disease: A case-control study. J Dent 2018; 77: 51-58.
Ohmure H, Oikawa K, Kanematsu K et al. Influence of experimental esophageal acidification on sleep bruxism: a randomized trial. J Dent Res 2011; 90: 665-671.
Beddis H, Pemberton M, Davies S. Sleep bruxism: an overview for clinicians. Br Dent J 2018; 225: 497-501.
Author information
Authors and Affiliations
Contributions
Drafting of the manuscript and final approval of the manuscript was carried out by Tom Thayer. Review of the manuscript was done by Rahat Ali.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0.© The Author(s) 2022
About this article
Cite this article
L. T. Thayer, M., Ali, R. The dental demolition derby: bruxism and its impact - part 1: background. Br Dent J 232, 515–521 (2022). https://doi.org/10.1038/s41415-022-4143-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-022-4143-8
This article is cited by
-
Neuromuscular and occlusion analysis to evaluate the efficacy of three splints on patients with bruxism
BMC Oral Health (2023)
-
Relationships between tooth wear, bruxism and temporomandibular disorders
British Dental Journal (2023)