Abstract
Aims To determine patient awareness of periodontal health, dentine hypersensitivity and tooth wear, and their impact on oral health quality of life in patients attending NHS practices in South West England.
Method In this cross-sectional, multi-centre epidemiological study 814 adult NHS patients completed an oral health questionnaire and then underwent a clinical examination. Pocket probing depths (mm), gingival recession (mm), gingival bleeding (yes/no), dentine hypersensitivity (Schiff score, and yes/no) and tooth wear (basic erosive wear examination score) were measured.
Results Participants were regular dental attenders, with good oral hygiene practices and a low prevalence of periodontal disease (probing depth of 4 mm or more) (25%). For all conditions assessed, self-reported data and clinical indices were significantly positively associated, with the strongest associations being seen for dentine hypersensitivity and the weakest for tooth wear. Periodontal disease and dentine hypersensitivity were significantly associated with all four patient-reported measures of oral health quality of life studied.
Conclusion This NHS patient population is well cared for and educated with respect to their oral health. The findings confirm the negative impact of periodontal disease and dentine hypersensitivity, and identifies the need to increase awareness of the signs and symptoms of tooth wear.
Similar content being viewed by others
Key points
-
Reports the oral health of the cohort of NHS patients in this study was very good and participants were aware of their periodontal condition and of dentine hypersensitivity.
-
Highlights that both periodontitis and dentine hypersensitivity have a negative effect on quality of life.
-
Suggests tooth wear is prevalent but inadequately recognised in this population.
Introduction
Periodontal disease in the UK is prevalent with 45% of adults showing some signs of periodontitis in the latest UK Adult Dental Health Survey (ADHS) and 9% showing signs of severe disease.1 These figures are similar to US and global estimates of prevalence; globally 46% of adults aged 35-44 have at least one tooth with periodontal pocketing of 4 mm or more2 and 11% of adults have severe periodontitis with periodontal pocketing of 6 mm or more,3 while in the US the National Health and Nutrition Examination Surveys (NHANES) between 2009 and 2012 showed that 46% of those aged 30 or over had periodontitis and 8% had severe disease.4
Periodontal disease has been shown to be important to sufferers; studies demonstrate that it has a negative impact on the quality of life through its effect on oral function, psychological wellbeing and capacity to cause pain, with evidence suggesting that the impact increases with the severity of disease.5 Functional effects include difficulties encountered chewing or speaking clearly,6 while consequences of periodontal disease such as halitosis7 are associated with negative effects on psychological wellbeing such as being self-conscious and unable to relax.8 Pain can result from the exposure of root dentine following treatment9 and, in periodontal patients receiving supportive care, almost 50% reported pain from dentine sensitivity; this self-reported sensitivity correlated with higher VAS scores following airblast and tactile stimulation of periodontally affected teeth.10 In addition to the effects that periodontal disease has on the oral cavity, there is also now good evidence that it is associated with a large number of systemic diseases.11
While there are successful treatments for periodontal disease,12 it is, in the majority of individuals, totally preventable. Microbial biofilms accumulate in areas of the oral cavity where they are less likely to be disturbed by external physical factors such as areas where teeth are crowded13 or sheltered areas such as the gingival crevice, periodontal pocket and interdental regions.14 Initially, gingivitis characterised by bleeding on brushing or probing occurs. This reversible condition progressing to periodontitis is characterised by a loss of tooth-supporting alveolar bone, and ultimately tooth loss in the majority of cases.15,16 While progress of the disease follows this linear progression in many individuals, evidence also exists that indicates in some individuals it progresses in a more random manner, with sites undergoing bursts of activity and bone destruction, but may then remain unchanged for periods of time.17 Gingivitis has been shown to be prevalent, with 54% of UK adults shown to have gingival bleeding in the most recent ADHS.1 This figure is relatively low, however, due to adults improving their personal care and being generally more informed about their oral health. By contrast, worldwide the prevalence of gingivitis remains high with gingival bleeding detected in 90-100% of individuals.18
Given the impact of periodontal disease on quality of life, increasing evidence for its negative effect on general health, and the fact that periodontitis is preventable and gingivitis reversible, why do rates of periodontal disease remain high?
It is recognised that periodontal disease can be present but painless and with few symptoms.5 Therefore, sufferers may not even know that they have the condition. Indeed, studies that have compared self-reported gingival bleeding and self-reported periodontal disease with clinically-determined gingival bleeding or periodontitis have shown under-reporting on the side of the patient.19,20 Similar under-reporting has been observed with other oral conditions such as dentine hypersensitivity (DH) where figures obtained via questionnaire were lower than participant response to clinical evaluation.21 DH arising when dentine is exposed and dentine tubules patent to the pulp22 is known to be associated with periodontal disease. Chabanski et al.,23 for example, demonstrated that 98% of periodontal patients had sensitivity. However, DH is also linked to healthy gingival recession in patients not susceptible to periodontitis, with 42% of young adults in Europe reporting DH.21 The increased prevalence of non-cervical carious lesions (NCCL) due to the rise in erosive tooth wear increases the prevalence of both healthy and periodontally-associated recession, with 29% of young adults demonstrating a BEWE score of 2 or 3,24 and 77% of adults showing tooth wear-exposing dentine in the UK ADHS 2009.1
The ability of patients to determine if they have periodontal disease or other oral conditions such as DH or tooth wear is important, as recognition of the symptoms of the disease enables the individual to seek help/treatment. Furthermore, if they are able to recognise early stage symptoms then they will be able to access treatment sooner with better outcomes. If we as clinicians can understand what patients understand about their oral health we may be able to improve oral hygiene advice and target messages about what to be aware of, what should be treated by a dentist and the consequences of lack of treatment.
The data presented here aimed to determine NHS patients' awareness of various aspects of their oral health and to compare patient-reported scores for specific conditions with those measured clinically to see how similar they were. It also examined the association of specific conditions with some indicators of oral health quality of life. These data were collected as part of a larger study that investigated the prevalence of periodontal disease, its association with other oral conditions and potential underlying risk factors, the findings of which will be presented separately.
Methods
The study was a cross-sectional, epidemiological, multi-centre study of adult patients attending NHS dental practices that were taking part in the in the Dental Foundation Training Scheme in the South West of England. The study was approved by the Health Research Authority and the North West-Preston NHS research ethics committee (IRAS ID: 218303; REC reference 16/NW/0850), and carried out according to the principles of the Declaration of Helsinki following good clinical practice (GCP) guidelines. Data were collected by newly qualified dental foundation trainees (DFTs) who received training in research methods, GCP and the clinical indices that were used in the study. Calibration to ensure consistency of scoring between dentists was undertaken on a training day, with trainers and DFTs scoring each of the clinical conditions in adult volunteers who had given written informed consent. Where there was a variation in the scoring of a particular index between the DFT and trainer, further training was given and the DFT asked to score the condition in another volunteer. For each dentist, training was undertaken until competency in scoring was achieved.
Study participants were patients attending an NHS practice for a routine appointment or check-up with the DFT. Patients that gave written informed consent were enrolled in the study and their eligibility assessed by the DFT. Eligible participants were adults aged 18 or over, in good general health and had a minimum of ten teeth. Patients who had used analgesics in the previous four hours or required antibiotic cover were excluded from the study.
Enrolled participants were asked to complete the study questionnaire. The study questionnaire was based on one used previously in a European study.21 It included questions to capture patient-reported oral hygiene practices, such as frequency of toothbrushing (number of times per day), attendance at a dentist (number of times per year), use of fluoridated toothpaste (yes/no). There were also questions about patients' perception of their oral health (yes/no) such as: 'Do you have wobbly teeth? Do your gums bleed when you brush your teeth? Do you think you have signs of tooth wear? Have your gums shrunk or receded? Can you can see more of your tooth than you could when you were younger?'. In addition, there were four oral health quality of life questions derived from DHEQ-15,25 which participants scored on a seven-point scale from strongly disagree to strongly agree: 1) Having sensations in my teeth takes a lot of the pleasure out of eating and drinking; 2) it takes a long time to finish some foods and drinks because of sensations in my teeth; 3) I have to change the way I eat or drink certain things; 4) I have to be careful how I breathe on a cold day.
Following completion of the questionnaire, the DFT completed a clinical examination of the buccal and lingual surfaces of all teeth except the third molars. Clinical measurements recorded were periodontal pocket probing depths (mm), recession (mm), the presence or absence of gingival bleeding, the presence or absence of exposed dentine, erosive tooth wear (basic erosive wear examination),26 and DH following an air blast using the examiner scored Schiff index and patient response (yes/no) (Table 1).27 Dental implants, teeth with orthodontic brackets and any with crowns and bridges were excluded.
Statistical analysis
The data were transferred to SPSS (version 23) for analysis. The percentage of individuals with a specific clinical condition/questionnaire response are presented, missing values were excluded. The analyses in Table 2 relate questionnaire variables, which are binary, to logically corresponding clinical indices, which are generally expressed as whole-mouth maximum scores and are ordinal or binary. We report the proportions positive by questionnaire and by clinical examination, with the corresponding sensitivity and specificity. The strength of association is characterised by the generalised Mann-Whitney statistic U/mn which generalises sensitivity and specificity. All these are displayed with 95% confidence intervals. The comparison between Schiff score and patient-reported DH at tooth level is restricted to participants who declared they had sensitive teeth. Here, no CIs or p-values are calculated due to non-independence. In Table 3, associations are between ordinal variables and are characterised by Spearman's rho.
Results
Data were collected between February and July 2017 at 28 NHS dental practices. The study recruited 814 participants aged 18 to 92 with a relatively even distribution of participants aged 20-29 through to 60-69, and a male to female ratio of 2:3. All 814 participants completed the questionnaire and then underwent a clinical exam.
Clinical assessments demonstrated evidence of periodontitis (pocket probing depths of 4 mm or more) in 28% of participants, and 11% had probing depths 6 mm or more in at least one site indicating severe periodontal disease. Evidence of bleeding on probing as a marker of inflammation and active periodontal disease was observed in 76% of participants. Almost 90% of participants showed some evidence of gingival recession and clinically relevant recession, described as 4 mm or more, affected 27% of participants. Minimal tooth wear, maximum basic erosive wear examination (BEWE) score of 1, was detected in 24% of participants, while 54% of participants had at least one BEWE score of 2 (clinically relevant tooth wear) and 20% of participants had a maximum BEWE score of 3 (severe tooth wear). There was a 98% agreement between having a Schiff score of one or more and claiming to have DH. Some DH (Schiff score 1) was seen in 33% of participants while clinically relevant DH scores (Schiff score of 2 or 3), were recorded in 24% of participants, with scores of three most frequently seen in the premolar and first molar regions in both arches and on the incisors in the lower arch. Dentine exposure was observed in 75% of participants on at least one tooth.
Participant-reported oral health assessed by questionnaire demonstrated that the majority of participants rated their oral health as good, very good or excellent (72%), with only 5% rating their oral health as poor. Oral hygiene practices were generally good with the majority of participants (82%) brushing at least twice daily, 17% brushing once a day and only 1% brushing less than once daily. Participants were also regular dental attenders, 87% having visited the dentist within the past year and 95% within the past two years.
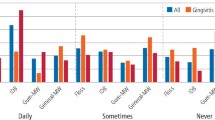
Participant responses to more specific aspects of their oral health are shown in Figure 1. When asked about gingival bleeding, 29% of participants indicated their gums bled, 44% of whom had used home treatments for bleeding gums and 66% of whom had spoken to their dentist about their bleeding gums. Wobbly teeth were reported by 9% of participants and 36% of that cohort said this affected their eating. Eleven percent of participants indicated they had bad breath, 88% of whom confirmed that this concerned them. Gingival recession was reported by 62% of participants and 54% reported they had tooth wear, however a further 29% did not know whether they had tooth wear or not. Of those who indicated they had tooth wear, only 14% had tried over the counter tooth wear products, although 43% had talked to their dentist about tooth wear. Almost half of the study participants (45%) also reported they had sensitive teeth, with 74% having used a home-use sensitivity treatment and 67% having previously spoken to their dentist about their sensitive teeth. Teeth identified by participants most frequently as sensitive were the upper right first molar, the upper left first molar, the lower left first molar and the lower incisors.
For each oral condition considered there was overall highly significant evidence for positive agreement between self-reported and clinically-determined presence of the condition (Table 2), with p <0.001 in all analyses. However, the degree of patient awareness varied widely between different parameters. A major factor in this was that some conditions, notably bleeding, are grossly under-reported by patients whereas gum shrinkage is grossly over-reported. This impacts on both the sensitivity, which expresses their ability to identify that they have the condition, and the specificity, indicating their ability to identify that they do not.
Thus, for both probing depth and recession depth, slightly over a quarter of participants had a maximum reading of 4 mm or greater. The prevalence of self-reported gum shrinkage was much higher than this, 62.1%, whereas only 8.8% of participants said they had wobbly teeth. Accordingly, for subjective gum shrinkage, the sensitivity is high and the specificity is low, but this pattern is reversed for tooth mobility. The degree of difference between the maximum probing depths for those with and without tooth mobility, expressed as U/mn = 0.785, is much higher than in the analysis for gum shrinkage (0.604).
Three-quarters of participants had bleeding on probing at one or more sites, whereas only 30% claimed to experience bleeding on brushing. Consequently, they were better at identifying when they didn't have a bleeding issue (specificity 89.3%) than when they did (sensitivity 35.5%), with a mediocre U/mn of 0.610.
For DH, the proportion of participants who had any Schiff score of 1 or higher (57.6%) was only moderately higher than the proportion who reported having DH (45.2%). Consequently, they were rather better at identifying when they didn't have DH (specificity 78.4%) than when they did (sensitivity 62.2%). Furthermore, in an analysis at tooth level with a Schiff threshold 2 +, based on all teeth from participants who reported sensitivity, there was a similar degree of under-reporting, leading here to a very low sensitivity (24.4%). This and the greatly reduced U/mn indicate that, although they were reasonably aware of whether they had a DH issue, patients were much less able to identify which teeth were affected.
Tooth wear was substantially under-reported, with sensitivity and specificity both mediocre at 59% and a relatively weak degree of separation of scores between those who do and do not report wear (U/mn = 0.591).
Participants responses with respect to the impact of their oral health on their quality of life are shown in Figure 2. Oral health had a negative impact on the pleasure of eating and drinking for 27% of participants, 30% reported modifying their eating and drinking behaviour as a result of their oral health. Furthermore, 17% said that they were slow to eat and drink, and 20% altered the way they breathed on a cold day due to problems with their mouths or teeth.
The associations between clinical variables and self-reported indicators of oral health quality of life are shown in Table 3. Greater periodontal pocketing and higher DH scores were associated with poorer oral health quality of life as assessed by all four self-reported measures, although the associations, while highly significant, were not that strong. Gingival bleeding and recession were less associated with the oral health quality of life measures assessed in this study, but some positive associations were detected; by contrast there were no associations with tooth wear.
Discussion
This study recruited 814 adults aged 18 or over attending routine dental appointments at 28 NHS dental practices across the South West of England. This sample size is similar to the number recruited from the general population by mailshot of addresses selected at random in the South West in the latest UK Adult Dental Health Survey (ADHS).28 The ages of the study participants ranged from 18 to 92 years old, and there was a relatively even distribution of participants across the age ranges up to age 70; a distribution in line with UK population data from 2017 which showed approximately similar numbers of individuals at all ages up to 55, followed by a steady decline which was slow to age 65 and increasing thereafter.29
The findings of the present study demonstrated that participants were regular NHS dental attenders with generally good self-reported oral hygiene practices which were evidenced by their relatively low levels of periodontal disease as compared to the prevalence observed in the ADHS 2009.1 As might be expected in regular dental attendees, participants were aware of conditions affecting their teeth and gums, with significant associations between clinical indices for all oral conditions and the corresponding patient self-reported data. However, the strength of the association varied. Patients were most aware of DH, periodontal disease and gingival recession, but less aware of gingival bleeding and tooth wear. While there was good agreement between clinically-determined and self-reported DH, the proportion of participants self-reporting DH was 13% lower than the proportion who responded positively following clinical stimulation, a finding that is similar to that reported by West et al.21 and may reflect the fact that DH pain is transient. Participants in the present study also had some ability to identify the teeth that were most commonly sensitive, although the strength of this association was much weaker, indicating that it may be difficult for patients to precisely identify which tooth is responsible for the pain. This highlights the need for a clinical assessment of DH, as home use treatments are becoming increasingly targeted to individual teeth30,31 and it is therefore important that the patient can recognise which tooth to treat.
In the present study, there was also good agreement between the proportion of participants who reported mobile or wobbly teeth and the prevalence of severe periodontitis detected clinically. This is probably a result of the cumulative nature of periodontal disease and the regular dental attendance of participants in this study. Those with periodontal disease were likely to have been diagnosed by their dentist and advised of the sequelae of bone loss leading to mobile teeth over time. Periodontal disease was also associated with gingival recession. Similar significant associations between clinically-determined periodontitis and self-reported indicators of periodontal disease have been demonstrated previously.19,20,32 However, in the study by Airila-Månsson20 where figures were given for prevalence, periodontitis was significantly under-reported by study participants; only 1.2% reported periodontal problems, while 17.1% had pocket probing depths of 5 mm or more. Similarly, in the present study, while 25% of participants had pocket probing depths of 4 mm or more, only 9% reported wobbly teeth. However, clinical experience shows patients do not find mobility of teeth easy to detect, unless the tooth is grade III, due to the lack of a fixed reference, unlike the bodily movement of a tooth when it changes position. A much higher number of individuals (29%) reported bleeding gums indicative of active disease, suggesting they were aware of their periodontal health.
Under-reporting by participants was also observed for gingival recession in the current study, even though there was a relatively strong association between the self-reported and clinically-determined data, suggesting that patients were aware of the condition. Nearly two-thirds of participants reported their gums had receded, however 90% of participants had some evidence of recession on clinical examination. This difference is likely to be because recession is not easily visible to the participant in the mirror; that is, if they look in a mirror as it is to the dentist during a clinical exam where lingual and palatal surfaces in particular are easier to view. Similar under-reporting has been detected previously in a study where only 16.7% of participants reported an awareness of gingival recession, even though over 60% showed generalised recession and tooth abrasion,33 a discrepancy that is considerably larger than that found in the present study. Interestingly, in the present study, clinically-detected gingival recession was also significantly associated with both self-reported wobbly teeth and tooth wear; the diversity of the correlations supports the idea that some of the recession present may be associated with poor oral hygiene and periodontal disease, while other areas of recession may be a result of excessive toothbrushing in the quest for excellent oral hygiene which can also result in tooth wear.34
The difference between self-reported and clinically detected gingival bleeding was the greatest observed in this study, only a third of study participants confirming they had gingival bleeding while the prevalence for clinical bleeding on probing was 75%. As almost half of the participants had used home treatments for bleeding gums and just over half had spoken to their dentist about their bleeding gums, participants appeared to be aware that bleeding gums should be treated. Similar findings, where self-reported bleeding of the gums was lower than clinically-detected gingival bleeding has been found previously.19 In both studies, the clinical exam was capable of detecting minor bleeding which a participant might not notice, particularly if it was only at the very back of the mouth where participants are not able to see it, or minimal and less obvious when mixed with toothpaste and saliva. In both studies, however, self-reported and clinically-detected gingival bleeding were significantly positively correlated but since, in the present study, the association was less strong than that observed between self-reported and clinically-determined periodontitis, gingival recession and DH, it seems that participant awareness was less for gingival bleeding than these conditions.
In the study reported here, participants were least aware of their tooth wear with only a weak, albeit significant, association observed between self-reported and clinically-determined tooth surface loss. This was also the question that yielded the largest number of 'don't know' responses, highlighting that this, of all the conditions, is the one that this population of dentally aware and orally healthy participants were least sure about, even though almost half of them had asked their dentist about tooth wear. This lack of awareness of tooth wear may reflect the lack of impact tooth wear had on quality of life in the present study, with tooth wear being the only condition not associated with any of the four quality of life indicators. This lack of impact on quality of life is indicative that tooth wear in the early stages does not cause any problems that are obvious to patients. However, given that tooth wear can result in the exposure of dentine and DH,35 and DH was strongly associated with all oral quality of life measures recorded in this study, it is clear that if patients are unaware of their tooth wear and allow it to worsen, an impact on their quality of life is likely in the future. Similar to DH, periodontitis was also strongly associated with all four quality of life measures and as both were also the conditions that participants were most aware of, perhaps not surprisingly this suggests that patients are more aware of oral conditions that affect daily activities such as eating and drinking. These data supports previous findings which have shown that both periodontal disease and DH have a negative impact on quality of life.36,37 Bleeding on probing was associated with three, and gingival recession two, of the oral health quality of life measures recorded here, however these associations were not strong and it is difficult to rationalise with current knowledge.
The number of participants in the study indicate the strength of the study. However, there were some study limitations. While the foundation dentists were excellent at completing paperwork for participants they enrolled in the study, unfortunately accurate information regarding how many participants had been approached is not available, study information was distributed to patients on arrival at the practice and the dentist was not necessarily told how many patients had declined the information. However, there was a good age and gender distribution which was similar to UK population data, although whether the participant population was biased or not cannot be determined. The data were also collected by a large number of dentists, which is hard to avoid when collecting such a large dataset. However, the dentists were all at the same stage of their dental career, having recently qualified and within their first year of dental practice, and they received the same training at the same time; thus, the dentists had exactly the same experience of using the scoring indices. Competence with the indices was tested in a clinical situation with trainers, the study dentists being assessed against the scores generated by the trainers and re-trained where their scores deviated until agreement was reached and the study dentists were confident. In addition, the study dentists were supported in their practices at the start of the study to ensure they were scoring correctly and completing study paperwork as indicated.
The study could also have asked more oral health quality of life questions, but when designing the study there were concerns about the length of time the questionnaire would take to complete and that it should not be too daunting for study participants. In interpreting the findings, it is important to realise that questionnaire responses about oral conditions and clinical measurements are, unavoidably, not identical; bleeding on brushing and bleeding on probing, for example. Also, when patients grossly over- or under-estimate prevalence, it must be recognised that this is relative to the threshold chosen for positivity of the clinical condition, for example whether this is 4 mm or 3 mm. Nevertheless, while changing the threshold for maximum gingival recession to more than 3 mm greatly affects the proportion positive for gingival recession, it only slightly affects sensitivity and specificity and cannot affect U/mn.
Conclusions
Taken together, the findings from the study reported here demonstrate that the periodontal health, oral health in general, and self-reported oral hygiene practices of these regular NHS dentist attendees was good and the positive, mostly strong associations between self-reported and clinically-detected conditions indicates that these patients were educated and aware of their oral health. This is particularly encouraging as this study cohort were NHS patients. However, the study did identify that, even in this group, participants were not particularly confident about tooth wear and not able to readily determine if they had it or not. This is an important finding as tooth wear is increasing and the cumulative effects of tooth wear over time can negatively impact function, appearance and be responsible for DH in the long-term. Dentists need to regularly assess the presence (or absence) of tooth wear and, if present, inform the patients about its aetiology and appearance. GDPs also need to focus on prevention of tooth wear and patient awareness. Even in this population, who were being well looked after, tooth wear and the other oral conditions examined were under-reported, emphasising the need for dental professionals to continually try to educate their patients about their teeth and gums and the consequences of failing to look after them.
References
White D, Pitts N, Steele J, Sadler K, Chadwick B. Disease and related disorders - a report from the Adult Dental Health Survey 2009. 2011. Available at https://files.digital.nhs.uk/publicationimport/pub01xxx/pub01086/adul-dent-heal-surv-summ-them-the2-2009-rep4.pdf (accessed August 2019).
World Health Organisation. WHO Global Oral Health Data Bank. Available at http://www.dent.niigata-u.ac.jp/prevent/perio/contents.html (accessed August 2019).
Kassebaum N J, Bernabé E, Dahiya M, Bhandari B, Murray C J, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res 2014; 93: 1045-1053.
Eke P I, Dye B A, Wei L et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol 2015; 86: 611-622.
Buset S L, Walter C, Friedmann A, Weiger R, Borgnakke W S, Zitzmann N U. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J Clin Periodontol 2016; 43: 333-344.
Ng S K, Leung W K. Oral health-related quality of life and periodontal status. Community Dent Oral Epidemiol 2006; 34: 114-122.
Silva M F, Cademartori M G, Leite F R M, López R, Demarco F F, Nascimento G G. Is periodontitis associated with halitosis? A systematic review and meta-regression analysis. J Clin Periodontol 2017; 44: 1003-1009.
Lu H X, Chen X L, Wong M, Zhu C, Ye W. Oral health impact of halitosis in Chinese adults. Int J Dent Hyg 2017; 15: e85-e92.
Lin Y H, Gillam D G. The prevalence of root sensitivity following periodontal therapy: a systematic review. Int J Dent 2012; 407023. DOI: 10.1155/2012/407023.
Goh V, Corbet E F, Leung W K. Impact of dentine hypersensitivity on oral health-related quality of life in individuals receiving supportive periodontal care. J Clin Periodontol 2016; 43: 595-602.
Loos B G. Periodontal medicine: work in progress! J Clin Periodontol 2016; 43: 470-471.
Graziani F, Karapetsa D, Alonso B, Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol 2000 2017; 75: 152-188.
Chung C H, Vanarsdall R L, Cavalcanti E A, Baldinger J S, Lai C H. Comparison of microbial composition in the subgingival plaque of adult crowded versus non-crowded dental regions. Int J Adult Orthodon Orthognath Surg 2000; 15: 321-330.
Silva N, Abusleme L, Bravo D et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci 2015; 23: 329-355.
Löe H, Theilade E, Jensen S B. Experimental gingivitis in man. J Periodontol 1965; 36: 177-187.
Löe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol 1986; 13: 431-445.
Sokransky S S, Haffajee A D, Goodson J M, Lindhe J. New concepts of destructive periodontal disease. J Clin Periodontol 1984; 11: 21-32.
Petersen P E, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol 2005; 76: 2187-2193.
Buhlin K, Gustafsson A, Andersson K, Håkansson J, Klinge B. Validity and limitations of self-reported periodontal health. Community Dent Oral Epidemiol 2002; 30: 431-437.
Airila-Månsson S, Bjurshammar N, Yakob M, Söder B. Self-reported oral problems, compared with clinical assessment in an epidemiological study. Int J Dent Hyg 2007; 5: 82-86.
West N X, Sanz M, Lussi A, Bartlett D, Bouchard P, Bourgeois D. Prevalence of dentine hypersensitivity and study of associated factors: a European population-based cross-sectional study. J Dent 2013; 41: 841-851.
Absi E G, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol 1987; 14: 280-284.
Chabanski M B, Gillam D G, Bulman J S, Newman H N. Clinical evaluation of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department: a pilot study. J Oral Rehabil 1997; 24: 666-672.
Bartlett D W, Lussi A, West N X, Bouchard P, Sanz M, Bourgeois D. Prevalence of tooth wear on buccal and lingual surfaces and possible risk factors in young European adults. J Dent 2013; 41: 1007-1013.
Machuca C, Baker S R, Sufi F, Mason S, Barlow A, Robinson P G. Derivation of a short form of the Dentine Hypersensitivity Experience Questionnaire. J Clin Periodontol 2013; 41: 46-51.
Bartlett D, Ganss C, Lussi A. Basic Erosive Wear Examination (BEWE): a new scoring system for scientific and clinical needs. Clin Oral Investig 2008; 12 (Spec Iss): S65-S68.
Schiff T, Dotson M, Cohen S, De Vizio W, McCool J, Volpe A. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: a twelve-week clinical study. J Clin Dent 1994; 5 (Spec Iss): 87-92.
O'Sullivan I, Lader D, Beavan-Seymour C, Chenery V, Fuller E, Sadler K. Foundation Report: Adult Dental Health Survey 2009 (Technical information). 2011. Available at https://files.digital.nhs.uk/publicationimport/pub01xxx/pub01086/adul-dent-heal-surv-summ-them-foun-2009-re14.pdf (accessed August 2019).
Office for National Statistics. Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid-2017. 2018. Available at https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2017#nearly-12-million-uk-residents-aged-65-years-and-over (accessed August 2019).
West N, Newcombe R G, Hughes N et al. A 3-day randomised clinical study investigating the efficacy of two toothpastes, designed to occlude dentine tubules, for the treatment of dentine hypersensitivity. J Dent 2013; 41: 187-194.
Papas A, Singh M, Magnuson M, Miner M, Sagel P, Gerlach R. Randomised controlled trial evaluation use of two different oxalate products in adults with recession-associated dentin hypersenstivity. Compend Contin Educ Dent 2016; 37: 26-31.
Blizniuk A, Ueno M, Zaitsu T, Kawaguchi Y. Association between self-reported and clinical oral health status in Belarusian adults. J Investig Clin Dent 2017; 8: e12206.
Shetty A, Bhandary R, Thomas B. Awareness on gingival recession and its association to risk factors: an epidemiological study. Research 2014; 1: 1268.
Addy M, Hunter M L. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J 2003; 53 (Spec Iss): 177-186.
West N, Seong J, Davies M. Dentine hypersensitivity. Monogr Oral Sci 2014; 25: 108-122.
Needleman I, McGrath C, Floyd P, Biddle A. Impact of oral health on the life quality of periodontal patients. J Clin Periodontol 2004; 31: 454-457.
Bekes K, Hirsch C. What is known about the influence of dentine hypersensitivity on oral health-related quality of life? Clin Oral Investig 2013; 17 (Spec Iss): S45-S51.
Acknowledgements
We would like to acknowledge the Foundation Dentists that were involved in the data collection: Richard Newton, Farima Mehrabi, Adam Auckland, Louise Griffith, Claire Mills, James Wade, Sarah Al-Kutubi, Jason Mallon, Reena Rishi, Sravya Makam, Ahmad Zeitoni, Mahir Hussain, Sarah Jacobs, Zoe Harding, Chris Yelland, Geethanjali Senthikumaran, Euan Stocker, Nisa Shah, Jessica Naylor, Rachel Moorey, Roxanne Tabatabal, Bethan Jones, Matthew Smith, Matthew Stephens, Scott Bowerman, Blue Coupe, Romana Linkova, Jeong Lee Min, Amy Bray.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out by the Clinical Trials Unit at Bristol Dental Hospital and sponsored by the University of Bristol. Support for the study was received from GSK consumer healthcare.
Rights and permissions
About this article
Cite this article
Midwood, I., Davies, M., Newcombe, R. et al. Patients' perception of their oral and periodontal health and its impact: a cross-sectional study in the NHS. Br Dent J 227, 587–593 (2019). https://doi.org/10.1038/s41415-019-0721-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-019-0721-9
This article is cited by
-
Patient-reported understanding and dentist-reported management of periodontal diseases - a survey: do you know what gum disease is?
British Dental Journal (2023)
-
Evaluation of gingival displacement methods in terms of periodontal health at crown restorations produced by digital scan: 1-year clinical follow-up
Lasers in Medical Science (2021)