Abstract
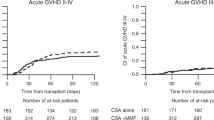
Post-transplant cyclophosphamide plus calcineurin inhibitor (CNI)(tacrolimus or cyclosporine A) plus mycophenolate mofetil (PTCy/TAC or CSA/MMF) and anti-thymocyte globulin plus CNI (tacrolimus or cyclosporine A) plus methotrexate (ATG/TAC or CSA/MTX) are common graft-versus-host disease (GVHD) prophylaxis regimens. We compared the two regimens in patients with acute myeloid leukemia (AML) undergoing allogeneic transplantation from matched siblings or unrelated donors. 402 received PTCy/TAC or CSA/MMF and 5648 received ATG/TAC or CSA/MTX. Patients in the PTCy-based group were younger (48.7 vs. 51.5 years, p = 0.024) and there was a higher frequency of patient cytomegalovirus seropositivity and female donor to male patient combination in this group (77.8% vs. 71.8%, p = 0.009 and 18.4% vs. 14.4%, p = 0.029, respectively). More patients in the PTCy-based group received reduced-intensity conditioning (51.5% vs. 41%, p < 0.0001). No differences were observed in the incidence of acute GVHD grade II–IV and III–IV (21.2% vs. 20.4%, p = 0.92 and 8.1% vs. 6%, p = 0.1) or 2-year total and extensive chronic GVHD (33.7% vs. 30%, p = 0.09 and 10.7% vs. 11.2%, p = 0.81) between the groups. In the multivariate analysis, all transplant outcomes did not differ between the groups. PTCy/CNI/MMF and ATG/CNI/MTX are alternative regimens for GVHD prophylaxis in AML patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
AN, ML, FC, and MM had full access to all study data (available upon data-specific request).
References
Zeiser R, Blazar BR. Acute graft-versus-host disease—biologic process, prevention, and therapy. N. Engl J Med. 2017;377:2167–79.
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Prim. 2023;9:27.
Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N. Engl J Med. 2017;377:2565–79.
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e159.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. ATG-Fresenius Trial Group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Cell Therapy Transplant Canada. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2020;7:e100–e111.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–11.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Anti-lymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl J Med. 2016;374:43–53.
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93.
Nagler A, Labopin M, Blaise D, Raiola AM, Corral LL, Bramanti S, et al. Non-T-depleted haploidentical transplantation with post-transplant cyclophosphamide in patients with secondary versus de novo AML in first complete remission: a study from the ALWP/EBMT. J Hematol Oncol. 2023;16:58.
Sanz J, Galimard JE, Labopin M, Afanasyev B, Angelucci E, Ciceri F, et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13:46.
Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134:892–9.
Lorentino F, Labopin M, Ciceri F, Vago L, Fleischhauer K, Afanasyev B, et al. Post-transplantation cyclophosphamide GvHD prophylaxis after hematopoietic stem cell transplantation from 9/10 or 10/10 HLA-matched unrelated donors for acute leukemia. Leukemia. 2021;35:585–94.
Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53.
Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol Oncol. 2020;13:87.
Soltermann Y, Heim D, Medinger M, Baldomero H, Halter JP, Gerull S, et al. Reduced dose of post-transplantation cyclophosphamide compared to ATG for graft-versus-host disease prophylaxis in recipients of mismatched unrelated donor hematopoietic cell transplantation: a single-center study. Ann Hematol. 2019;98:1485–93.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomized phase 2 trial with a non-randomized contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–e143.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N. Engl J Med. 2023;388:2338–48.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Kanate AS, Nagler A, Savani B. Summary of scientific and statistical methods, study endpoints and definitions for observational and registry-based studies in hematopoietic cell transplantation. Clin Hematol Int. 2019;2:2–4.
Andersen PK, Klein JP, Zhang MJ. Testing for center effects in multi-center survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
McCafrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–414.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/.
Al Malki MM, Tsai NC, Palmer J, Mokhtari S, Tsai W, Cao T, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv. 2021;5:2650–9.
Bacigalupo A, Jones R. PTCy the “new” standard for GVHD prophylaxis. Blood Rev. 2023;62:101096.
Leick M, Bin Chen Y. A glimpse into what happens after PTCy. Blood. 2022;139:479–81.
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Onco. 2018;11:40.
Carnevale-Schianca F, Caravelli D, Gallo S, Becco P, Paruzzo L, Poletto S, et al. Post-transplant cyclophosphamide and tacrolimus–mycophenolate mofetil combination governs GVHD and immunosuppression need, reducing late toxicities in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. J Clin Med. 2021;10:1173.
Mehta RS, Saliba, et al. Post-transplantation cyclophosphamide versus tacrolimus and methotrexate graft-versus-host disease prophylaxis for HLA-matched donor transplantation. Transpl Cell Ther. 2022;28:695.e1–695.e10.
Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot MR, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial. Blood Adv. 2022;6:3378–85.
Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40:356–68.
Modi D, Kondrat K, Kim S, Deol A, Ayash L, Ratanatharathorn V, et al. Post-transplant cyclophosphamide versus thymoglobulin in HLA-mismatched unrelated donor transplant for acute myelogenous leukemia and myelodysplastic syndrome. Transpl Cell Ther. 2021;27:760–7.
Berro M, Rivas M, Trucco J, Paganini I, Ravchina I, Kusminsky G. Post-transplant cyclophosphamide demonstrates lower non-relapse mortality and better graft-versus-host disease/relapse-free survival compared with antithymocyte globulin in unrelated donor hematopoietic stem cell transplantation. A single-center experience. Bone Marrow Transpl. 2021;56:986–8.
Dachy F, Furst S, Calmels B, Pagliardini T, Harbi S, Bouchacourt B, et al. GVHD prophylaxis with post-transplant cyclophosphamide results in lower incidence of GVHD and allows faster immunosuppressive treatment reduction compared to antithymocyte globulin in 10/10 HLA-matched unrelated allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2023;58:1179–81.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transpl. 2016;22:1037–42.
Massoud R, Gagelmann N, Fritzsche-Friedland U, Zeck G, Heidenreich S, Wolschke C, et al. Comparison of immune reconstitution between anti-T-lymphocyte globulin and posttransplant cyclophosphamide as acute graft-versus-host disease prophylaxis in allogeneic myeloablative peripheral blood stem cell transplantation. Haematologica. 2022;107:857–67.
Tang, Liu L, Li Z, Dong T, Wu T, Niu Q, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin in allogeneic hematopoietic stem cell transplantation from unrelated donors: a systematic review and meta-analysis. Front Oncol. 2023;13:1071268.
Greinix HT, Eikema DJ, Koster L, Penack O, Yakoub-Agha I, Montoto S, et al. Improved outcome of patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: an EBMT mega-file study. Haematologica. 2022;107:1054–63.
Turki AT, Tsachakis-Mück N, Leserer S, Crivello P, Liebregts T, Betke L, et al. Impact of CMV reactivation on relapse of acute myeloid leukemia after HCT is dependent on disease stage and ATG. Blood Adv. 2022;6:28–36.
Acknowledgements
We thank all the EBMT centers and national registries for contributing patients to this study (Supplementary appendix material). We also thank the data managers for their excellent work.
Author information
Authors and Affiliations
Contributions
AN wrote the manuscript, designed the study, and interpreted the data. ML and MM designed the study, performed the statistical analyses, interpreted the data, and edited the manuscript. RS helped with writing the first draft of the manuscript. TS, RMH, LG, US, AR, SM, AK, JP, PD, TGD, EF, GH, MS, CCL, AS, EB, and FC reviewed the manuscript and provided clinical data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The scientific boards of the ALWP of the EBMT approved this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagler, A., Labopin, M., Swoboda, R. et al. Post-transplant cyclophosphamide, calcineurin inhibitor, and mycophenolate mofetil compared to anti-thymocyte globulin, calcineurin inhibitor, and methotrexate combinations as graft-versus-host disease prophylaxis post allogeneic stem cell transplantation from sibling and unrelated donors in patients with acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02284-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02284-5