Abstract
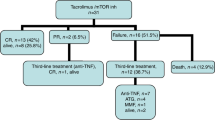
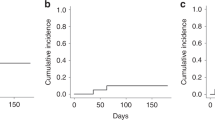
Acute graft-versus-host disease (aGVHD) is the major treatment-related complication after stem-cell transplantation (SCT) from unrelated donors. The proteasome-inhibitor bortezomib was added to GVHD prevention regimens with initial promise. However, two randomized studies failed to show efficacy. We explored the addition of carfilzomib, s second-generation proteasome inhibitor (20 mg/m2, intravenously on days +1 and +2) to cyclosporine/methotrexate backbone in 26 patients after SCT from unrelated donors. We compared outcomes to historical group of 100 patients given cyclosporine/methotrexate alone. Median follow-up was 34 months. There was no difference between the groups in engraftment or toxicities. The cumulative incidence of aGVHD grade II–IV, 6 months post transplant was 11% (95% CI, 4–32) and 39% (95% CI, 30–50), respectively (P = 0.01). The cumulative incidence of chronic GVHD, 2 years post transplant, was 49% (95% CI, 32–75) and 41% (95% CI, 33–52), respectively (P = 0.98). Three-year non-relapse mortality was 11% (95% CI, 4–33) and 18% (95% CI, 12–27, P = 0.45) while 3-year relapse rates were 8% (95% CI, 2–29) and 26% (95% CI, 18–36), respectively (P = 0.06). Three-year survival was 81% (95%CI, 66–96) and 56% (95% CI, 46–66), respectively (P = 0.05). In conclusion, carfilzomib with cyclosporine/methotrexate is safe, may reduce aGVHD, and possibly improve survival after unrelated donor SCT. These initial observations merit further study in larger comparative studies. ClinicalTrial.gov NCT01991301.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35.
Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73:1729–34.
Al-Homsi AS, Feng Y, Duffner U, Al Malki MM, Goodyke A, Cole K, et al. Bortezomib for the prevention and treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2016;44:771–7.
Mohty M, Brissot E, Savani BN, Gaugler B. Effects of bortezomib on the immune system: a focus on immune regulation. Biol Blood Marrow Transplant. 2013;19:1416–20.
Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–9.
Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–8.
Koreth J, Kim HT, Lange PB, Bindra B, Reynolds CG, Chammas MJ, et al. A bortezomib-based regimen offers promising survival and graft-versus-host disease prophylaxis in myeloablative HLA-mismatched and unrelated donor transplantation: a phase ii trial. Biol Blood Marrow Transplant. 2015;21:1907–13.
Koreth J, Kim HT, Lange PB, Poryanda SJ, Reynolds CG, Rai SC, et al. Bortezomib-based immunosuppression after reduced-intensity conditioning hematopoietic stem cell transplantation: randomized phase II results. Haematologica. 2018;103:522–30.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–43.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Pidala J, Jaglowski S, Im A, Chen G, Onstad L, Storer B, et al. Carfilzomib for treatment of refractory chronic graft-versus-host disease: a chronic GVHD consortium pilot phase ii trial. Biol Blood Marrow Transplant. 2020;26:278–84.
Pawarode A, Couriel DR, Braun T, Magenau JM, Riwes MM, Parkin BL, et al. Phase 1 study of carfilzomib in allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2016;22:S19eS481.
Yerushalmi R, Shem-Tov N, Danylesko I, Shouval R, Nagler A, Shimoni A. The combination of cyclosporine and mycophenolate mofetil is less effective than cyclosporine and methotrexate in the prevention of acute graft-versus host disease after stem-cell transplantation from unrelated donors. Am J Hematol. 2017;92:259–68.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55:1114–25.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assos. 1958;53:457–81.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Funding
The study was supported by a research grant from Onyx/Amgen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimoni, A., Shem-Tov, N., Yerushalmi, R. et al. Carfilzomib combined with cyclosporine and methotrexate for the prevention of graft-versus-host disease after allogeneic stem-cell transplantation from unrelated donors. Bone Marrow Transplant 56, 451–456 (2021). https://doi.org/10.1038/s41409-020-01044-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01044-5