Abstract
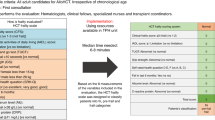
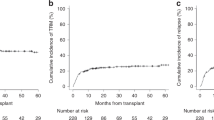
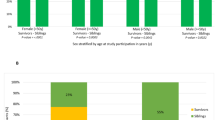
Older patients are at increased risk for complications and death following allogeneic hematopoietic cell transplantation (allo-HCT). Traditional transplant-specific prognostic indices such as hematopoietic cell transplant comorbidity index (HCT-CI) may not capture all underlying geriatric vulnerabilities, and in-depth evaluation by a geriatrician prior to transplant may not always be available. We hypothesize that routine pretransplant interdisciplinary clinical assessment may uncover prognostically important geriatric deficits. Using an institutional database of 457 adults aged 60 years and older who underwent first allo-HCT for hematological malignancies from 2010 to 2017, we examined the prognostic impact of pretransplant deficits in geriatric domains of function, mobility, mood, medication, nutrition, and relevant biochemical markers. We found that impairment in instrumental activities of daily living (IADL) was associated with reduced survival through increased nonrelapse mortality (NRM, HR = 1.82; 95% CI, 1.04–3.19). The combination of IADL impairment with either HCT-CI/age index or disease risk index readily stratified NRM and overall survival, respectively. In addition, we found that even mild renal dysfunction adversely impacted survival in older transplant patients. Our findings establish important geriatric vulnerabilities in older patients prior to allo-HCT and may provide an entry point for prospective, interventional trials to improve their outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Giralt SA. Hematopoietic cell transplantation for older adults. In: Korc-Grodzicki B, Tew WP, editors. Handbooks of geriatrics oncology—practical guide to caring for the older cancer patient. New York, NY: Demos Medical Publishing; 2017. p. 241–54.
Pulte D, Jansen L, Castro FA, Brenner H. Changes in the survival of older patients with hematologic malignancies in the early 21st century. Cancer. 2016;122:2031–40.
McClune BL, Weisdorf DJ, Pedersen TL, da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87.
Elsawy M, Sorror M. Up-to-date tools for risk assessment before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51:1283–300.
Sorror ML, Storb RF, Sandmaier B, Maziarz RT, Pulsipher MA, Maris MB, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32:3249–56.
Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Ustun C, Warlick ED, et al. Predictive value of disease risk comorbidity index for overall survival after allogeneic hematopoietic transplantation. Blood Adv. 2019;3:230–6.
Vaughn JE, Storer BE, Armand P, Raimondi R, Gibson C, Rambaldi A, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biol Blood Marrow Transplant. 2015;21:1418–24.
Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101:1426–33.
D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides. 2017. http://www.cibmtr.org. Accessed 15 Apr 2019.
Hahn T, McCarthy PL Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, use unrelated donors. J Clin Oncol. 2013;31:2437–49.
Sorror ML, Sandmaier B, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–83.
Kennedy VE, Muffly LS. Assessment of older adult candidates for allogeneic hematopoietic cell transplantation: updates and remaining questions. Expert Rev Hematol. 2019;12:99–106.
Rosko A, Artz A. Aging: treating the older patient. Biol Blood Marrow Transplant. 2017;23:193–200.
Artz A. Biologic vs physiologic age in the transplant candidate. Hematology. 2016;2016:99–105.
Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–9.
Deschler B, Ihorst G, Schnitzler S, Bertz H, Finke J. Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: a prospective risk factor and serial assessment analysis. Bone Marrow Transplant. 2018;53:565–75.
Nawas MT, Andreadis C, Martin TG, Wolf JL, Ai WZ, Kaplan LD et al. Limitation in patient-reported function is associated with inferior survival in older adults undergoing autologous hematopoietic cell transplantation. Biology Blood Marrow Transplant. 2019. https://doi.org/10.1016/j.bbmt.2019.01.028.
Lin RJ, Shahrokni A, Dahi PB, Jakubowski AA, Devlin S, Maloy MA, et al. Pretransplant comprehensive geriatric assessment in hematopoietic cell transplantation: a single center experience. Bone Marrow Transplant. 2018;53:1184–7.
Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–63.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
the Panel B. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–46.
DuMontier C, Liu MA, Murillo A, Hshieh T, Houman J, Soiffer R, et al. Functional, survival, and care utilization among older adults with hematologic malignancies. J Am Geriatr Soc. 2019;67:889–97.
Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2016;22:651–7.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Fein JA, Shimoni A, Labopin M, Shem-Tov N, Yerushalmi R, Magen H, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia. 2018;32:1787–94.
Shouval R, de Jong CN, Fein J, Broers A, Danylesko I, Shimoni A, et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol Blood Marrow Transplant. 2018;24:1685–91.
Lin RJ, Hilden PD, Elko TA, Dahi PB, Shahrokni A, Jakubowski AA, et al. Burden and impact of multifactorial geriatric syndromes in allogeneic hematopoietic cell transplantation for older adults. Blood Adv. 2019;3:12–20.
Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36:2326–47.
Acknowledgements
This research was supported in part by National Institutes of Health award numbers P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. RJL received support from the New York State Empire Clinical Research Investigator Program (ECRIP) and the Elsa U. Pardee Foundation for Cancer Research. This work was presented in part at the 60th American Society of Hematology Annual Meeting and Exposition, December 1–4, 2018, San Diego, CA; and at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting, May 31-June 4, 2019, Chicago, IL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
SAG reports research funding from Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi Biotec, and Takeda and serves as a consultant for Amgen, Actinium, Celgene, Johnson & Johnson, Jazz Pharmaceuticals, Miltenyi Biotec, Takeda, Novartis, Kite Pharma, and Spectrum Pharmaceuticals. MAP reports honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has also received research support for clinical trials from Incyte and Miltenyi Biotec. CSS has served as a paid consultant on advisory boards for: Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, a Gilead Company and GSK. He has received research funds for investigator-initiated trials from: Juno Therapeutics and Sanofi-Genzyme. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lin, R.J., Elko, T.A., Devlin, S.M. et al. Impact of geriatric vulnerabilities on allogeneic hematopoietic cell transplantation outcomes in older patients with hematologic malignancies. Bone Marrow Transplant 55, 157–164 (2020). https://doi.org/10.1038/s41409-019-0654-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0654-6
This article is cited by
-
Transplantation and Cellular Therapy for Older Adults—The MSK Approach
Current Hematologic Malignancy Reports (2024)
-
HCT frailty scale for younger and older adults undergoing allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2023)
-
Impact of hematopoietic cell transplant frailty scale on transplant outcome in adults
Bone Marrow Transplantation (2023)
-
Multidisciplinary Approach to Older Adults with Hematologic Malignancies—a Paradigm Shift
Current Hematologic Malignancy Reports (2022)
-
Pitfalls and Successes in Trials in Older Transplant Patients with Hematologic Malignancies
Current Oncology Reports (2022)