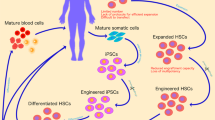
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is a standard therapeutic intervention for hematological malignancies and several monogenic diseases. However, this approach has limitations related to lack of a suitable donor, graft-versus-host disease and infectious complications due to immune suppression. On the contrary, autologous HSCT diminishes the negative effects of allogeneic HSCT. Despite the good efficacy, earlier gene therapy trials with autologous HSCs and viral vectors have raised serious safety concerns. However, the CRISPR/Cas9-edited autologous HSCs have been proposed to be an alternative option with a high safety profile. In this review, we summarized the possibility of CRISPR/Cas9-mediated autologous HSCT as a potential treatment option for various diseases supported by preclinical gene-editing studies. Furthermore, we discussed future clinical perspectives and possible clinical grade improvements of CRISPR/cas9-mediated autologous HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Qian L, Wu Z, Shen J. Advances in the treatment of acute graft-versus-host disease. J Cell Mol Med. 2013;17:966–75. https://doi.org/10.1111/jcmm.12093.
Barriga F, Ramírez P, Wietstruck A, Rojas N. Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res. 2012;45:307–16.
Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the american society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2015;21:1863–9. https://doi.org/10.1016/j.bbmt.2015.07.032.
Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. https://doi.org/10.1146/annurev.immunol.25.022106.141606.
Norkin M, Wingard JR. Recent advances in hematopoietic stem cell transplantation. F1000Res. 2017;6:870. https://doi.org/10.12688/f1000research.11233.1.
Ballen KK, Koreth J, Chen YB, Dey BR, Spitzer TR. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119:1972–80. https://doi.org/10.1182/blood-2011-11-354563.
Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–48. https://doi.org/10.1056/NEJMsa1311707.
Kohne E. Hemoglobinopathies: clinical manifestations, diagnosis, and treatment. Dtsch Arztebl Int. 2011;108:532–40. https://doi.org/10.3238/arztebl.2011.0532.
Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. https://doi.org/10.1182/blood-2011-06-364265.
Lee SE, Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48:587–92. https://doi.org/10.1038/bmt.2012.187.
Psatha N, Karponi G, Yannaki E. Optimizing autologous cell grafts to improve stem cell gene therapy. Exp Hematol. 2016;44:528–39. https://doi.org/10.1016/j.exphem.2016.04.007.
Henig I, Zuckerman T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med J. 2014;5:e0028. https://doi.org/10.5041/RMMJ.10162.
Groeschel S, Kuhl JS, Bley AE, Kehrer C, Weschke B, Doring M, et al. Long-term outcome of allogeneic hematopoietic stem cell transplantation in patients with juvenile metachromatic leukodystrophy compared with nontransplanted control patients. JAMA Neurol. 2016;73:1133–40. https://doi.org/10.1001/jamaneurol.2016.2067.
Sessa M, Lorioli L, Fumagalli F, Acquati S, Redaelli D, Baldoli C, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–87. https://doi.org/10.1016/S0140-6736(16)30374-9.
De Ravin SS, Reik A, Liu PQ, Li L, Wu X, Su L, et al. Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat Biotechnol. 2016;34:424–9. https://doi.org/10.1038/nbt.3513.
Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377:1630–8. https://doi.org/10.1056/NEJMoa1700554.
Negre O, Eggimann AV, Beuzard Y, Ribeil JA, Bourget P, Borwornpinyo S, et al. Gene therapy of the beta-Hemoglobinopathies by Lentiviral Transfer of the beta(A(T87Q))-Globin Gene. Hum Gene Ther. 2016;27:148–65. https://doi.org/10.1089/hum.2016.007.
Braun CJ, Boztug K, Paruzynski A, Witzel M, Schwarzer A, Rothe M, et al. Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity. Sci Transl Med. 2014;6:227ra233. https://doi.org/10.1126/scitranslmed.3007280.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–9. https://doi.org/10.1038/nm1393.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9. https://doi.org/10.1126/science.1088547.
Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. https://doi.org/10.1038/4401123a.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–22. https://doi.org/10.1038/nature09328.
Zonari E, Desantis G, Petrillo C, Boccalatte FE, Lidonnici MR, Kajaste-Rudnitski A, et al. Efficient ex vivo engineering and expansion of highly purified human hematopoietic stem and progenitor cell populations for gene therapy. Stem Cell Rep. 2017;8:977–90. https://doi.org/10.1016/j.stemcr.2017.02.010.
Antony JS, Haque AKMA, Lamsfus-Calle A, Daniel-Moreno A, Mezger M, Kormann MSD. CRISPR/Cas9system: a promising technology for the treatment of inherited and neoplastic hematological diseases. Adv Cell Gene Ther. 2018;1:e10. https://doi.org/10.1002/acg2.10.
Gutierrez-Guerrero A, Sanchez-Hernandez S, Galvani G, Pinedo-Gomez J, Martin-Guerra R, Sanchez-Gilabert A, et al. Comparison of zinc finger nucleases versus CRISPR-specific nucleases for genome editing of the Wiskott-Aldrich Syndrome locus. Hum Gene Ther. 2018;29:366–80. https://doi.org/10.1089/hum.2017.047.
Fan Y, Chan JKY. Editing the genome ex vivo stem cell therapy. Curr Stem Cell Rep. 2018;4:338–45. https://doi.org/10.1007/s40778-018-0148-2.
Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–55. https://doi.org/10.1038/nbt.2842.
Chandrakasan S, Malik P. Gene therapy for hemoglobinopathies: the state of the field and the future. Hematol Oncol Clin North Am. 2014;28:199–216. https://doi.org/10.1016/j.hoc.2013.12.003.
Thein SL. The molecular basis of beta-thalassemia. Cold Spring Harb Perspect Med. 2013;3:a011700. https://doi.org/10.1101/cshperspect.a011700.
Nienhuis AW, Nathan DG. Pathophysiology and clinical manifestations of the beta-thalassemias. Cold Spring Harb Perspect Med. 2012;2:a011726. https://doi.org/10.1101/cshperspect.a011726.
Olivieri NF, Weatherall DJ. The therapeutic reactivation of fetal haemoglobin. Hum Mol Genet. 1998;7:1655–8.
May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. https://doi.org/10.1038/35017565.
Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N Engl J Med. 2018;378:1479–93. https://doi.org/10.1056/NEJMoa1705342.
Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–9. https://doi.org/10.1038/nature20134.
Hoban MD, Lumaquin D, Kuo CY, Romero Z, Long J, Ho M, et al. CRISPR/Cas9-mediated correction of the sickle mutation in human CD34 + cells. Mol Ther. 2016;24:1561–9. https://doi.org/10.1038/mt.2016.148.
DeWitt MA, Magis W, Bray NL, Wang T, Berman JR, Urbinati F, et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 2016;8:360ra134. https://doi.org/10.1126/scitranslmed.aaf9336.
Antony JS, Latifi N, Haque A, Lamsfus-Calle A, Daniel-Moreno A, Graeter S, et al. Gene correction of HBB mutations in CD34( + ) hematopoietic stem cells using Cas9 mRNA and ssODN donors. Mol Cell Pediatr. 2018;5:9. https://doi.org/10.1186/s40348-018-0086-1.
Vakulskas CA, Dever DP, Rettig GR, Turk R, Jacobi AM, Collingwood MA, et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat Med. 2018;24:1216–24. https://doi.org/10.1038/s41591-018-0137-0.
Liu Y, Yang Y, Kang X, Lin B, Yu Q, Song B, et al. One-step biallelic and scarless correction of a beta-thalassemia mutation in patient-specific iPSCs without drug selection. Mol Ther Nucleic Acids. 2017;6:57–67. https://doi.org/10.1016/j.omtn.2016.11.010.
Magis W, DeWitt MA, Wyman SK, Vu JT, Heo S-J, Shao SJ, et al. In vivo selection for corrected β-globin alleles after CRISPR/Cas9 editing in human sickle hematopoietic stem cells enhances therapeutic potential. bioRxiv 2018:432716. https://doi.org/10.1101/432716.
Cai L, Bai H, Mahairaki V, Gao Y, He C, Wen Y, et al. A universal approach to correct various HBB gene mutations in human stem cells for gene therapy of beta-thalassemia and sickle cell disease. Stem Cells Transl Med. 2018;7:87–97. https://doi.org/10.1002/sctm.17-0066.
Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, Zhang B, et al. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing. Sci Rep. 2016;6:23549. https://doi.org/10.1038/srep23549.
Bauer DE, Kamran SC, Orkin SH. Reawakening fetal hemoglobin: prospects for new therapies for the beta-globin disorders. Blood. 2012;120:2945–53. https://doi.org/10.1182/blood-2012-06-292078.
Traxler EA, Yao Y, Wang YD, Woodard KJ, Kurita R, Nakamura Y, et al. A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med. 2016;22:987–90. https://doi.org/10.1038/nm.4170.
Martyn GE, Wienert B, Yang L, Shah M, Norton LJ, Burdach J, et al. Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat Genet. 2018;50:498–503. https://doi.org/10.1038/s41588-018-0085-0.
Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527:192. https://doi.org/10.1038/nature15521.
Antoniani C, Meneghini V, Lattanzi A, Felix T, Romano O, Magrin E, et al. Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human beta-globin locus. Blood. 2018;131:1960–73. https://doi.org/10.1182/blood-2017-10-811505.
Ye L, Wang J, Tan Y, Beyer AI, Xie F, Muench MO, et al. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc Natl Acad Sci. 2016;113:10661–5. https://doi.org/10.1073/pnas.1612075113.
Hacein-Bey Abina S, Gaspar HB, Blondeau J, Caccavelli L, Charrier S, Buckland K, et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–63. https://doi.org/10.1001/jama.2015.3253.
Candotti F. Advances of gene therapy for primary immunodeficiencies. F1000Res 2016;5. https://doi.org/10.12688/f1000research.7512.1.
Alzubi J, Pallant C, Mussolino C, Howe SJ, Thrasher AJ, Cathomen T. Targeted genome editing restores T cell differentiation in a humanized X-SCID pluripotent stem cell disease model. Sci Rep. 2017;7:12475. https://doi.org/10.1038/s41598-017-12750-4.
Hacein-Bey-Abina S, Pai SY, Gaspar HB, Armant M, Berry CC, Blanche S, et al. A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med. 2014;371:1407–17. https://doi.org/10.1056/NEJMoa1404588.
Genovese P, Schiroli G, Escobar G, Tomaso TD, Firrito C, Calabria A, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–40. https://doi.org/10.1038/nature13420.
Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25:1298–306. https://doi.org/10.1038/nbt1353.
Chang CW, Lai YS, Westin E, Khodadadi-Jamayran A, Pawlik KM, Lamb LS Jr., et al. Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting. Cell Rep. 2015;12:1668–77. https://doi.org/10.1016/j.celrep.2015.08.013.
Schiroli G, Ferrari S, Conway A, Jacob A, Capo V, Albano L, et al. Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1. Sci Transl Med. 2017;9. https://doi.org/10.1126/scitranslmed.aan0820.
Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–9. https://doi.org/10.1038/s41586-018-0326-5.
Almasbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602. https://doi.org/10.1155/2016/5474602.
Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–5. https://doi.org/10.1073/pnas.0813101106.
Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017;27:154–7. https://doi.org/10.1038/cr.2016.142.
Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9. https://doi.org/10.1126/scitranslmed.aaj2013.
Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173:1439–53 e1419. https://doi.org/10.1016/j.cell.2018.05.013.
Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2017;23:2255–66. https://doi.org/10.1158/1078-0432.CCR-16-1300.
Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–7. https://doi.org/10.1038/nature21405.
Sather BD, Romano Ibarra GS, Sommer K, Curinga G, Hale M, Khan IF, et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med. 2015;7:307ra156. https://doi.org/10.1126/scitranslmed.aac5530.
Gschweng E, De Oliveira S, Kohn DB. Hematopoietic stem cells for cancer immunotherapy. Immunol Rev. 2014;257:237–49. https://doi.org/10.1111/imr.12128.
Bortin MM, Bach FH, van Bekkum DW, Good RA, van Rood JJ. 25th anniversary of the first successful allogeneic bone marrow transplants. Bone Marrow Transplant. 1994;14:211–2.
Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–10. https://doi.org/10.1056/NEJMoa1300662.
Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52. https://doi.org/10.1146/annurev.immunol.26.021607.090326.
Gurung P, Kanneganti TD. Autoinflammatory skin disorders: the inflammasomme in focus. Trends Mol Med. 2016;22:545–64. https://doi.org/10.1016/j.molmed.2016.05.003.
Beer HD, Contassot E, French LE. The inflammasomes in autoinflammatory diseases with skin involvement. J Invest Dermatol. 2014;134:1805–10. https://doi.org/10.1038/jid.2014.76.
Sand J, Haertel E, Biedermann T, Contassot E, Reichmann E, French LE, et al. Expression of inflammasome proteins and inflammasome activation occurs in human, but not in murine keratinocytes. Cell Death & Dis. 2018;9:24. https://doi.org/10.1038/s41419-017-0009-4.
Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. 2016;8:550–63. https://doi.org/10.1159/000446825.
Pohl K, Hayes E, Keenan J, Henry M, Meleady P, Molloy K, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999–1009. https://doi.org/10.1182/blood-2014-02-555268.
Laval J, Ralhan A, Hartl D. Neutrophils in cystic fibrosis. Biol Chem. 2016;397:485–96. https://doi.org/10.1515/hsz-2015-0271.
Yin H, Song CQ, Suresh S, Kwan SY, Wu Q, Walsh S, et al. Partial DNA-guided Cas9 enables genome editing with reduced off-target activity. Nat Chem Biol. 2018;14:311–6. https://doi.org/10.1038/nchembio.2559.
Nasri M, Mir P, Dannenmann B, Amend D, Skroblyn T, Xu Y, et al. Fluorescent labeling of CRISPR/Cas9 RNP for gene knockout in HSPCs and iPSCs reveals an essential role for GADD45b in stress response. Blood Adv. 2019;3:63–71. https://doi.org/10.1182/bloodadvances.2017015511.
Hamilton N, Sabroe I, Renshaw SA. A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models. F1000Res. 2018;7:594. https://doi.org/10.12688/f1000research.14507.2.
Wang J, Exline CM, DeClercq JJ, Llewellyn GN, Hayward SB, Li PW, et al. Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors. Nat Biotechnol. 2015;33:1256–63. https://doi.org/10.1038/nbt.3408.
Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic stem cell gene therapy: progress and lessons learned. Cell Stem Cell. 2017;21:574–90. https://doi.org/10.1016/j.stem.2017.10.010.
Cleyrat C, Girard R, Choi EH, Jeziorski E, Lavabre-Bertrand T, Hermouet S, et al. Gene editing rescue of a novel MPL mutant associated with congenital amegakaryocytic thrombocytopenia. Blood Adv. 2017;1:1815–26. https://doi.org/10.1182/bloodadvances.2016002915.
Diez B, Genovese P, Roman-Rodriguez FJ, Alvarez L, Schiroli G, Ugalde L, et al. Therapeutic gene editing in CD34( + ) hematopoietic progenitors from Fanconi anemia patients. EMBO Mol Med. 2017;9:1574–88. https://doi.org/10.15252/emmm.201707540.
Li SJ, Luo Y, Zhang LM, Yang W, Zhang GG. Targeted introduction and effective expression of hFIX at the AAVS1 locus in mesenchymal stem cells. Mol Med Rep. 2017;15:1313–8. https://doi.org/10.3892/mmr.2017.6131.
Ohmori T, Nagao Y, Mizukami H, Sakata A, Muramatsu SI, Ozawa K, et al. CRISPR/Cas9-mediated genome editing via postnatal administration of AAV vector cures haemophilia B mice. Sci Rep. 2017;7:4159. https://doi.org/10.1038/s41598-017-04625-5.
Huai C, Jia C, Sun R, Xu P, Min T, Wang Q, et al. CRISPR/Cas9-mediated somatic and germline gene correction to restore hemostasis in hemophilia B mice. Hum Genet. 2017;136:875–83. https://doi.org/10.1007/s00439-017-1801-z.
Dreyer AK, Hoffmann D, Lachmann N, Ackermann M, Steinemann D, Timm B, et al. TALEN-mediated functional correction of X-linked chronic granulomatous disease in patient-derived induced pluripotent stem cells. Biomaterials. 2015;69:191–200. https://doi.org/10.1016/j.biomaterials.2015.07.057.
Merling RK, Sweeney CL, Chu J, Bodansky A, Choi U, Priel DL, et al. An AAVS1-targeted minigene platform for correction of iPSCs from all five types of chronic granulomatous disease. Mol Ther. 2015;23:147–57. https://doi.org/10.1038/mt.2014.195.
Sweeney CL, Zou J, Choi U, Merling RK, Liu A, Bodansky A, et al. Targeted repair of CYBB in X-CGD iPSCs requires retention of intronic sequences for expression and functional correction. Mol Ther. 2017;25:321–30. https://doi.org/10.1016/j.ymthe.2016.11.012.
Flynn R, Grundmann A, Renz P, Hanseler W, James WS, Cowley SA, et al. CRISPR-mediated genotypic and phenotypic correction of a chronic granulomatous disease mutation in human iPS cells. Exp Hematol. 2015;43:838–48 e833. https://doi.org/10.1016/j.exphem.2015.06.002.
De Ravin SS, Li L, Wu X, Choi U, Allen C, Koontz S, et al. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci Transl Med. 2017;9. https://doi.org/10.1126/scitranslmed.aah3480.
Li L, Krymskaya L, Wang J, Henley J, Rao A, Cao LF, et al. Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol Ther. 2013;21:1259–69. https://doi.org/10.1038/mt.2013.65.
Li C, Guan X, Du T, Jin W, Wu B, Liu Y, et al. Inhibition of HIV-1 infection of primary CD4+T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J Gen Virol. 2015;96:2381–93. https://doi.org/10.1099/vir.0.000139.
Xu L, Yang H, Gao Y, Chen Z, Xie L, Liu Y, et al. CRISPR/Cas9-mediated CCR5 ablation in human hematopoietic stem/progenitor cells confers HIV-1 resistance in vivo. Mol Ther. 2017;25:1782–9. https://doi.org/10.1016/j.ymthe.2017.04.027.
Acknowledgements
This work was financed by the research funding programs Jürgen Manchot Stiftung, Fortüne Tübingen (N°. 2412-0-0; N°. 2485-0-0), and the University Children’s Hospital of Tübingen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daniel-Moreno, A., Lamsfus-Calle, A., Raju, J. et al. CRISPR/Cas9-modified hematopoietic stem cells—present and future perspectives for stem cell transplantation. Bone Marrow Transplant 54, 1940–1950 (2019). https://doi.org/10.1038/s41409-019-0510-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0510-8
This article is cited by
-
Stimuli-responsive nanoformulations for CRISPR-Cas9 genome editing
Journal of Nanobiotechnology (2022)