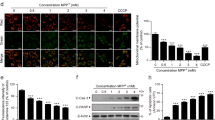
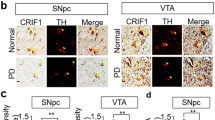
Abstract
Parkinson’s disease (PD) is a common neurodegenerative motor disorder characterized by a dramatic reduction in pars compacta of substantia nigra dopaminergic neurons and striatal dopamine (DA) levels. Mutations or deletions in the PARK7/DJ-1 gene are associated with an early-onset familial form of PD. DJ-1 protein prevents neurodegeneration via its regulation of oxidative stress and mitochondrial function as well as its roles in transcription and signal transduction. In this study, we investigated how loss of DJ-1 function affected DA degradation, ROS generation and mitochondrial dysfunction in neuronal cells. We showed that loss of DJ-1 significantly increased the expression of monoamine oxidase (MAO)-B but not MAO-A in both neuronal cells and primary astrocytes. In DJ-1-knockout (KO) mice, MAO-B protein levels in the substantia nigra (SN) and striatal regions were significantly increased. We demonstrated that the induction of MAO-B expression by DJ-1 deficiency depended on early growth response 1 (EGR1) in N2a cells. By coimmunoprecipitation omics analysis, we found that DJ-1 interacted with receptor of activated protein C kinase 1 (RACK1), a scaffolding protein, and thus inhibited the activity of the PKC/JNK/AP-1/EGR1 cascade. The PKC inhibitor sotrastaurin or the JNK inhibitor SP600125 completely inhibited DJ-1 deficiency-induced EGR1 and MAO-B expression in N2a cells. Moreover, the MAO-B inhibitor rasagiline inhibited mitochondrial ROS generation and rescued neuronal cell death caused by DJ-1 deficiency, especially in response to MPTP stimulation in vitro and in vivo. These results suggest that DJ-1 exerts neuroprotective effects by inhibiting the expression of MAO-B distributed at the mitochondrial outer membrane, which mediates DA degradation, ROS generation and mitochondrial dysfunction. This study reveals a mechanistic link between DJ-1 and MAO-B expression and contributes to understanding the crosslinks among pathogenic factors, mitochondrial dysfunction and oxidative stress in PD pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data supporting the findings in this study are available within the paper. Other transcriptome sequencing and mass spectrometry data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303.
Liu Y, Niu L, Liu X, Cheng C, Le W. Recent progress in non-motor features of Parkinson’s disease with a focus on circadian rhythm dysregulation. Neurosci Bull. 2021;37:1010–24.
Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–60.
Li S, Jia C, Li T, Le W. Hot topics in recent Parkinson’s disease research: where we are and where we should go. Neurosci Bull. 2021;37:1735–44.
Mao Q, Qin WZ, Zhang A, Ye N. Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease. Acta Pharmacol Sin. 2020;41:471–82.
Trist BG, Hare DJ, Double KL. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell. 2019;18:e13031.
Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909.
Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ. Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem Sci. 2021;46:329–43.
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–9.
Kumaran R, Vandrovcova J, Luk C, Sharma S, Renton A, Wood NW, et al. Differential DJ-1 gene expression in Parkinson’s disease. Neurobiol Dis. 2009;36:393–400.
Neves M, Graos M, Anjo SI, Manadas B. Modulation of signaling pathways by DJ-1: An updated overview. Redox Biol. 2022;51:102283.
Dekker M, Bonifati V, van Swieten J, Leenders N, Galjaard RJ, Snijders P, et al. Clinical features and neuroimaging of PARK7-linked parkinsonism. Mov Disord. 2003;18:751–7.
Dekker MC, Eshuis SA, Maguire RP, Veenma-van der Duijn L, Pruim J, Snijders PJ, et al. PET neuroimaging and mutations in the DJ-1 gene. J Neural Transm (Vienna). 2004;111:1575–81.
Xu X, Wang R, Hao Z, Wang G, Mu C, Ding J, et al. DJ-1 regulates tyrosine hydroxylase expression through CaMKKbeta/CaMKIV/CREB1 pathway in vitro and in vivo. J Cell Physiol. 2020;235:869–79.
Lu L, Sun X, Liu Y, Zhao H, Zhao S, Yang H. DJ-1 upregulates tyrosine hydroxylase gene expression by activating its transcriptional factor Nurr1 via the ERK1/2 pathway. Int J Biochem Cell Biol. 2012;44:65–71.
Ishikawa S, Taira T, Takahashi-Niki K, Niki T, Ariga H, Iguchi-Ariga SM. Human DJ-1-specific transcriptional activation of tyrosine hydroxylase gene. J Biol Chem. 2010;285:39718–31.
Zhong N, Kim CY, Rizzu P, Geula C, Porter DR, Pothos EN, et al. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem. 2006;281:20940–8.
Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–61.
Masato A, Plotegher N, Boassa D, Bubacco L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol Neurodegener. 2019;14:35.
Juarez Olguin H, Calderon Guzman D, Hernandez Garcia E, Barragan Mejia G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. 2016;2016:9730467.
Naoi M, Maruyama W, Inaba-Hasegawa K. Type A and B monoamine oxidase in age-related neurodegenerative disorders: their distinct roles in neuronal death and survival. Curr Top Med Chem. 2012;12:2177–88.
Tipton KF, Boyce S, O’Sullivan J, Davey GP, Healy J. Monoamine oxidases: certainties and uncertainties. Curr Med Chem. 2004;11:1965–82.
Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309.
Damier P, Kastner A, Agid Y, Hirsch EC. Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson’s disease? Neurology. 1996;46:1262–9.
Kang SS, Ahn EH, Zhang Z, Liu X, Manfredsson FP, Sandoval IM, et al. alpha-Synuclein stimulation of monoamine oxidase-B and legumain protease mediates the pathology of Parkinson’s disease. EMBO J. 2018;37:e98878.
Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–96.
Ren H, Fu K, Wang D, Mu C, Wang G. Oxidized DJ-1 interacts with the mitochondrial protein BCL-XL. J Biol Chem. 2011;286:35308–17.
Ren H, Fu K, Mu C, Zhen X, Wang G. L166P mutant DJ-1 promotes cell death by dissociating Bax from mitochondrial Bcl-XL. Mol Neurodegener. 2012;7:40.
Clarke N, Arenzana N, Hai T, Minden A, Prywes R. Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol Cell Biol. 1998;18:1065–73.
Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol. 2002;169:1922–9.
Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H, et al. Induction of COX-2-PGE2 synthesis by activation of the MAPK/ERK pathway contributes to neuronal death triggered by TDP-43-depleted microglia. Cell Death Dis. 2015;6:e1702.
Guo DK, Zhu Y, Sun HY, Xu XY, Zhang S, Hao ZB, et al. Pharmacological activation of REV-ERBalpha represses LPS-induced microglial activation through the NF-kappaB pathway. Acta Pharmacol Sin. 2019;40:26–34.
Sun HY, Wu J, Wang R, Zhang S, Xu H, Kaznacheyeva Е, et al. Pazopanib alleviates neuroinflammation and protects dopaminergic neurons in LPS-stimulated mouse model by inhibiting MEK4-JNK-AP-1 pathway. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-01030-1. Online ahead of print.
Gonzalez-Lopez E, Vrana KE. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J Neurochem. 2020;152:157–81.
Shih JC, Wu JB, Chen K. Transcriptional regulation and multiple functions of MAO genes. J Neural Transm (Vienna). 2011;118:979–86.
Wong WK, Ou XM, Chen K, Shih JC. Activation of human monoamine oxidase B gene expression by a protein kinase C MAPK signal transduction pathway involves c-Jun and Egr-1. J Biol Chem. 2002;277:22222–30.
Arige V, Agarwal A, Khan AA, Kalyani A, Natarajan B, Gupta V, et al. Regulation of monoamine oxidase B gene expression: key roles for transcription factors Sp1, Egr1 and CREB, and microRNAs miR-300 and miR-1224. J Mol Biol. 2019;431:1127–47.
Shih JC, Chen K. Regulation of MAO-A and MAO-B gene expression. Curr Med Chem. 2004;11:1995–2005.
Hijioka M, Inden M, Yanagisawa D, Kitamura Y. DJ-1/PARK7: A new therapeutic target for neurodegenerative disorders. Biol Pharm Bull. 2017;40:548–52.
Ou XM, Chen K, Shih JC. Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J Biol Chem. 2004;279:21021–8.
Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287:24195–206.
Hoffmann E, Ashouri J, Wolter S, Doerrie A, Dittrich-Breiholz O, Schneider H, et al. Transcriptional regulation of EGR-1 by the interleukin-1-JNK-MKK7-c-Jun pathway. J Biol Chem. 2008;283:12120–8.
Aggeli IK, Beis I, Gaitanaki C. ERKs and JNKs mediate hydrogen peroxide-induced Egr-1 expression and nuclear accumulation in H9c2 cells. Physiol Res. 2010;59:443–54.
Iyoda T, Zhang F, Sun L, Hao F, Schmitz-Peiffer C, Xu X, et al. Lysophosphatidic acid induces early growth response-1 (Egr-1) protein expression via protein kinase Cdelta-regulated extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle cells. J Biol Chem. 2012;287:22635–42.
Pagel JI, Deindl E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. Int J Mol Sci. 2012;13:13104–17.
Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–85.
Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–9.
Kappelmann M, Bosserhoff A, Kuphal S. AP-1/c-Jun transcription factors: regulation and function in malignant melanoma. Eur J Cell Biol. 2014;93:76–81.
Li JJ, Xie D. RACK1, a versatile hub in cancer. Oncogene. 2015;34:1890–8.
Lopez-Bergami P, Ronai Z. Requirements for PKC-augmented JNK activation by MKK4/7. Int J Biochem Cell Biol. 2008;40:1055–64.
Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected]. Mol Cell. 2005;19:309–20.
Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, et al. L166P mutant DJ-1, causative for recessive Parkinson’s disease, is degraded through the ubiquitin-proteasome system. J Biol Chem. 2003;278:36588–95.
Tan YY, Jenner P, Chen SD. Monoamine oxidase-B inhibitors for the treatment of Parkinson’s disease: past, present, and future. J Parkinsons Dis. 2022;12:477–93.
Nicotra A, Pierucci F, Parvez H, Senatori O. Monoamine oxidase expression during development and aging. Neurotoxicology. 2004;25:155–65.
Li L, Zhang CW, Chen GY, Zhu B, Chai C, Xu QH, et al. A sensitive two-photon probe to selectively detect monoamine oxidase B activity in Parkinson’s disease models. Nat Commun. 2014;5:3276.
Weng M, Xie X, Liu C, Lim KL, Zhang CW, Li L. The sources of reactive oxygen species and its possible role in the pathogenesis of Parkinson’s disease. Parkinsons Dis. 2018;2018:9163040.
Saller S, Kunz L, Berg D, Berg U, Lara H, Urra J, et al. Dopamine in human follicular fluid is associated with cellular uptake and metabolism-dependent generation of reactive oxygen species in granulosa cells: implications for physiology and pathology. Hum Reprod. 2014;29:555–67.
Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, et al. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med. 2001;30:924–31.
Cohen G, Kesler N. Monoamine oxidase and mitochondrial respiration. J Neurochem. 1999;73:2310–5.
Singh YP, Pandey A, Vishwakarma S, Modi G. A review on iron chelators as potential therapeutic agents for the treatment of Alzheimer’s and Parkinson’s diseases. Mol Divers. 2019;23:509–26.
Ben-Shachar D, Zuk R, Glinka Y. Dopamine neurotoxicity: inhibition of mitochondrial respiration. J Neurochem. 1995;64:718–23.
Sanchez-Rodriguez R, Munari F, Angioni R, Venegas F, Agnellini A, Castro-Gil MP, et al. Targeting monoamine oxidase to dampen NLRP3 inflammasome activation in inflammation. Cell Mol Immunol. 2021;18:1311–3.
da Costa CA. DJ-1: a newcomer in Parkinson’s disease pathology. Curr Mol Med. 2007;7:650–7.
Lev N, Barhum Y, Pilosof NS, Ickowicz D, Cohen HY, Melamed E, et al. DJ-1 protects against dopamine toxicity: implications for Parkinson’s disease and aging. J Gerontol A Biol Sci Med Sci. 2013;68:215–25.
Lev N, Ickowicz D, Barhum Y, Lev S, Melamed E, Offen D. DJ-1 protects against dopamine toxicity. J Neural Transm (Vienna). 2009;116:151–60.
Trudler D, Weinreb O, Mandel SA, Youdim MB, Frenkel D. DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J Neurochem. 2014;129:434–47.
Jiang HB, Jiang Q, Liu WH, Feng J. Parkin suppresses the expression of monoamine oxidases. J Biol Chem. 2006;281:8591–9.
Siddiqui A, Hanson I, Andersen JK. Mao-B elevation decreases parkin’s ability to efficiently clear damaged mitochondria: protective effects of rapamycin. Free Radic Res. 2012;46:1011–8.
Smith TS, Trimmer PA, Khan SM, Tinklepaugh DL, Bennett JP Jr. Mitochondrial toxins in models of neurodegenerative diseases. II: Elevated zif268 transcription and independent temporal regulation of striatal D1 and D2 receptor mRNAs and D1 and D2 receptor-binding sites in C57BL/6 mice during MPTP treatment. Brain Res. 1997;765:189–97.
Xie B, Wang C, Zheng Z, Song B, Ma C, Thiel G, et al. Egr-1 transactivates Bim gene expression to promote neuronal apoptosis. J Neurosci. 2011;31:5032–44.
Yu Q, Huang Q, Du X, Xu S, Li M, Ma S. Early activation of Egr-1 promotes neuroinflammation and dopaminergic neurodegeneration in an experimental model of Parkinson’s disease. Exp Neurol. 2018;302:145–54.
Cao J, Lou S, Ying M, Yang B. DJ-1 as a human oncogene and potential therapeutic target. Biochem Pharmacol. 2015;93:241–50.
Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89:867–82.
Ren H, Fu K, Mu C, Li B, Wang D, Wang G. DJ-1, a cancer and Parkinson’s disease associated protein, regulates autophagy through JNK pathway in cancer cells. Cancer Lett. 2010;297:101–8.
Kershner L, Welshhans K. RACK1 regulates neural development. Neural Regen Res. 2017;12:1036–9.
Ma J, Wu R, Zhang Q, Wu JB, Lou J, Zheng Z, et al. DJ-1 interacts with RACK1 and protects neurons from oxidative-stress-induced apoptosis. Biochem J. 2014;462:489–97.
Yamamori T, Mizobata A, Saito Y, Urano Y, Inanami O, Irani K, et al. Phosphorylation of p66shc mediates 6-hydroxydopamine cytotoxicity. Free Radic Res. 2011;45:342–50.
Do Van B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–78.
Zhu HY, He QJ, Yang B, Cao J. Beyond iron deposition: making sense of the latest evidence on ferroptosis in Parkinson’s disease. Acta Pharmacol Sin. 2021;42:1379–81.
Mo JS, Kim MY, Ann EJ, Hong JA, Park HS. DJ-1 modulates UV-induced oxidative stress signaling through the suppression of MEKK1 and cell death. Cell Death Differ. 2008;15:1030–41.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32170987, 31970966 and 32261133525), the Russian Science Foundation (No. 23-44-00054), the Natural Science Foundation of Jiangsu Province (BK20200213) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
HGR and BL designed the study. LLL, YH, and ZJZ performed most of the experiments. YQW, YWH, and BL performed some of the biochemical and cellular experiments. EK, JQD, and DKG analyzed the data. HGR drafted the paper. BL and GHW revised the paper. All authors read and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Ll., Han, Y., Zhang, Zj. et al. Loss of DJ-1 function contributes to Parkinson’s disease pathogenesis in mice via RACK1-mediated PKC activation and MAO-B upregulation. Acta Pharmacol Sin 44, 1948–1961 (2023). https://doi.org/10.1038/s41401-023-01104-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01104-8