Abstract
Prenatal dexamethasone exposure (PDE) can decrease maternal endogenous glucocorticoid level and induce testicular dysplasia in male offspring rats. In this study we investigated low level endogenous glucocorticoid-mediated testicular dysplasia in PDE offspring and elucidated the intrauterine epigenetic programming mechanisms. Pregnant rats were injected with dexamethasone (0.2 mg·kg−1·d−1, sc) on gestational day (GD) 9–20. The offspring rat blood and testis were collected after euthanasia on GD20, postnatal week (PW) 12 or PW28. We showed that PDE induced abnormal morphology of testis and significantly decreased the expression of testosterone synthesis-related genes as well as testosterone production before and after birth. Meanwhile, serum corticosterone, the expression and histone 3 lysine 14 acetylation (H3K14ac) of testicular insulin-like growth factor 1 (IGF1) were significantly decreased. After the pregnant rats were subjected to chronic stress for 2 weeks (PW10-12), serum corticosterone level was increased in the adult PDE offspring, and the above-mentioned other indicators were also improved. Cultured Leydig cells (TM3) were treated with corticosterone (62.5–500 nM) in vitro. We showed that corticosterone concentration-dependently inhibited glucocorticoid receptor α (GRα) and miR-124-3p expression, increased histone deacetylase 5 (HDAC5) expression, and decreased IGF1 H3K14ac level and the expression of IGF1/steroidogenic acute regulatory protein (StAR), suggesting that corticosterone at lower than physiological level (<500 nM) inhibited testosterone synthesis by reducing H3K14ac and the expression level of IGF1 through GRα/miR-124-3p/HDAC5 pathway. In conclusion, PDE can cause persistent inhibition of testosterone synthesis before and after birth in the offspring rats by low level of endogenous glucocorticoids.
Similar content being viewed by others
Introduction
It is known that basal glucocorticoids levels participate in the regulation of fetal multi-organ formation and development, and is a key factor in determining individual tissue morphological development and functional maturation after birth [1]. Our previous studies have shown that prenatal exposure to multiple xenobiotics (such as caffeine, nicotine and ethanol) can open placental barrier, leading to fetal overexposure to maternal glucocorticoids (e.g., cortisol in humans and corticosterone in rodents), it can cause intrauterine growth retardation (IUGR) and multi-organ dysfunction in offspring before and after birth, including testicular dysplasia [2,3,4]. It has also been reported that decreased corticosterone levels in adult rats can lead to decreased testicular spermatogenesis [5]. Therefore, the physiological endogenous glucocorticoids level plays an important role in the maintenance of testicular functional development. Our team and other researches found that prenatal dexamethasone exposure (PDE) can reduce corticosterone levels in mother and offspring [6, 7]. It also leads to abnormal testicular morphology and decreased testosterone secretion in offspring [8, 9]. However, can decreased intrauterine endogenous glucocorticoids induced by PDE cause the alteration of testosterone synthesis in offspring before and after birth? What is the intrauterine programming mechanism? These questions are still unclear.
Testosterone is known to be synthesized and secreted by testicular Leydig cells, and its biosynthesis is an ordered process that needs transporters and multiple steroid synthases. The steroidogenic acute regulatory protein (StAR) is a key transporter for testosterone synthesis, it can transfer cholesterol from the mitochondrial outer membrane to the intima, which is an important rate-limiting step in the synthesis process [10]. Insulin-like growth factor 1 (IGF1) is an important regulator of StAR, involved in the regulation of the testosterone synthesis in Leydig cells [11, 12]. It has recently been demonstrated that IGF1 null mice showed decreased StAR protein and blood testosterone levels [13]. Our previous study confirmed that prenatal caffeine exposure can decrease testicular local IGF1 expression by increasing intrauterine maternal endogenous glucocorticoids level, thereby inhibiting the expression of genes related to steroid synthesis; meanwhile, decreased basal glucocorticoids level after birth can promote expression of testicular IGF1 and steroidal synthase system, which exhibits “glucocorticoid-insulin-like growth factor 1 (GC-IGF1) axis negative programming” [14,15,16]. There is a significant knowledge gap about the low corticosterone level in utero caused by PDE can also induce the testicular “GC-IGF1 axis programming” alteration and its toxicological significance.
Epigenetic modification induced by multiple xenobiotics in utero can cause gene expression changes, and the epigenetic changes are closely related to disease [17, 18]. Arsenic exposure inhibited the steroidogenic gene expression by activating H3K9me3 status, which contributed to steroidogenic suppression in rat testis[19]. Exposure to bisphenol A in preimplantation embryo retards the development of testes by reducing histone acetylation level in StAR promoter to disrupt the testicular testosterone synthesis [20]. Histone deacetylases (HDACs) play an important role in many biological processes such as transcription, cell cycles, signal transduction, and gene expression regulation [21]. It has been reported that HDACs are involved in regulating histone modification and expression of IGF1 [22, 23]. MiRNAs, a kind of non-coding RNA molecules with 20–24 nucleotides and regulated by glucocorticoids, have been found to play an important role in regulating the expression of key genes. Increasing studies have found that histone acetylation changes of downstream target genes can be mediated by miRNAs through targeting Hdacs [24, 25]. Therefore, we speculate that lower than the physiological concentration of corticosterone caused by PDE may regulate histone acetylation and expression of IGF1 through miRNAs, resulting in altered programming of testicular development.
In this study, based on a stable PDE rat model established in our laboratory, we intended to confirm testicular developmental programming alteration by measuring testosterone synthesis and GC-IGF1 axis changes in the offspring before and after birth. Then, we verified the epigenetic programming mechanism of IGF1 expression changes caused by lower than the physiological concentration of corticosterone. This study illustrated the endogenous glucocorticoids programming mechanism of PDE-induced testicular dysplasia, comprehensively analyzed the short and long-term harm of PDE-induced testicular developmental programming and homeostasis changes, and provided a novel target and idea for early prevention and treatment strategies of testicular dysplasia induced by PDE.
Materials and methods
Chemicals and reagents
Dexamethasone was obtained from Shuanghe Pharmaceutical Company (Wuhan, China). Isoflurane (10019-360-40) was purchased from Baxter Healthcare Inc. (Deerfield, IL, Massachusetts, USA). The enzyme-linked immunosorbent assay (ELISA) kits for testosterone were obtained from the North Institute of Biological Technology (Beijing, China). The ELISA kits for corticosterone were obtained from the R&D Systems, Inc. (Minneapolis, MN, USA). The StAR antibody (No. 8449s) was purchased from CST, Inc. The 3β-HSD antibody (sc30820) was purchased from Santa Cruz, Inc. (Santa Cruz, CA, USA). The antibodies such as β-actin (AC004), HDAC5 (A0632), anti-acetyl histone 3 Lysine 14 (H3K14ac) (A7254) were purchased from Abclonal Technology Co., Ltd. (Wuhan, China). The antibodies of IgG (ab172730) and Ki67 (ab15580), and IGF1 recombinant protein (ab198569) were purchased from Abcam Technology Co., Ltd. (Cambridge, UK). DAB staining kit (GK347011) was obtained from Gene Tech Co., Ltd. (Shanghai, China). GRα plasmid (C05008) and luciferase reporter gene were purchased from Suzhou GenePharma Co., Ltd., miR-124-3p mimics (CN14379-1) and Hdac5 siRNA (SIGS0001597-1) were obtained from Guangzhou Ribo Co., Ltd. Lipofectamine 3000 reagent and Tizol reagent were obtained from Invitrogen (Carlsbad, CA, USA). Reverse transcription (RR047Q) and quantitative real-time polymerase chain reaction (qRT-PCR) kits (Q223) were purchased from Takara Biotechnology Co., Ltd. (Dalian, China). miDETECT A Track™ miRNA qRT-PCR Kit was purchased from Ribo Biotech Co., Ltd. (Guangzhou, China). All of the primers were synthesized by StARgene Biotech Co., Ltd. (Wuhan, China). DNA purification kits (Q5314) were purchased from TIANGEN Biotech Co., Ltd. (Beijing, China). Proteinase K (20 mg/kg) (ST533) was purchased from Kori Biotech Co., Ltd (Wuhan, China). BCA Protein Assay Kit (P0011) was purchased from Beyotime Biotechnology (Shanghai, China). The other reagents for experiments were of analytical grade.
Animals and treatment
Animal experiments were handled in the Center for Animal Experiments of Wuhan University (Wuhan, China), which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Wuhan University School of Medicine (permit number: 14016). All animal experimental procedures were approved by the Chinese Animal Welfare Committee.
Specific pathogen-free WiStAR rats [No: 2012-2014, certification No: 42000600002258, license No: SCXK (Hubei)] weighing 280 ± 20 g (males) and 200 ± 20 g (females) were purchased from the Experimental Center of the Hubei Medical Scientific Academy (Wuhan, China). The rats were housed in cages with wire-mesh floors in an air-conditioned room under standard conditions and allowed a normal diet. All rats were acclimated one week before experimentation. Two female rats were placed together with one male rat overnight in a cage for mating. On confirmation of mating by the appearance of sperm in a vaginal smear, the day was taken as the gestational day (GD) 0. As previously described [26], the PDE group was subcutaneously injected with 0.2 mg·kg−1·d−1 dexamethasone (Shuanghe Pharmaceutical Company, Wuhan, China) during GD9 to 20, respectively, and the control group was administered the same volume of saline. The flowchart of animal treatment is shown in Fig. 1.
From gestational day (GD) 9–20, pregnant WiStAR rats were subcutaneously injected with 0.2 mg·kg−1·d−1 dexamethasone. At postnatal week (PW) 10, two male rats were randomly selected from each litter to exposure to chronic stress for 2 weeks. Rats were anesthetized with 2% isoflurane and euthanized on GD20, PW12, and PW28.
The part of pregnant rats was anesthetized with isoflurane and euthanized on GD20 (n = 15 pregnant rats per group), and the blood sample was collected from the carotid artery. There were 30 pregnant rats in each group, and 15 pregnant rats in each group were randomly selected to be euthanized and sampled in GD20, and the remaining 15 rats were delivered normally. In the experiment, those dams with litter sizes of 8–14 were considered qualified, several pregnant rats that do not meet the requirements are usually eliminated, and 12 pregnant rats were finally guaranteed to be included in the next experiment. The male fetuses were decapitated immediately to obtain blood samples and testes, the blood samples of male fetal rats from each litter were pooled as one sample for analysis, and the three fetal rats’ testes randomly from each litter were randomly counted as one sample for gene and protein analysis. In addition, we randomly selected five fetal testes from different litters were fixed for morphological detection. The remaining 15 pregnant rats in each group were allowed to deliver normally. On postnatal day (PD) 1, the number of pups was normalized to 8 per litter (4 male and 4 female pups per litter, 8 litters per group). Two male offspring rats from each litter were selected and assigned to the studies at different postnatal time points or conditions (i.e., PW12, PW12 with chronic stress, and PW28), respectively. The chronic stress test was achieved by forced 5-min ice-water swimming per day from PW10 to PW12 [27]. In different postnatal time points, male rats were anesthetized with isoflurane and euthanized. Then, the blood sample from the carotid artery was collected, the serum was isolated, and the testes were rapidly collected. Except for portions of the male right testes (n = 5 per group) obtained from different litters were fixed for morphologic observations, and the other collected samples were immediately frozen at −80 °C for subsequent analysis. And more notably, when the adult testes have been fixed for 12 h, we punctured testicular tunica albuginea carefully with injection needles in different directions to speed up the permeability of paraformaldehyde and ensure its uniformity.
Analysis of serum samples
The blood samples were centrifuged at 1600 r/min for 10 min at 4 °C and the serum was collected. Serum corticosterone concentration was measured via corticosterone ELISA assay kit. The testicular homogenates were centrifuged at 3000 r/min for 10 min and the supernatants were collected for measuring intratesticular testosterone concentration. Intratesticular testosterone production and serum testosterone levels were measured via testosterone ELISA kit. The intra-assay and inter-assay coefficients of variation for testosterone were all not higher than 15%. All assay procedures followed the manufacturer’s protocols as our previous descriptions [9].
Hematoxylin-eosin (H&E) staining
The right testes were fixed in 4% paraformaldehyde overnight and processed with the paraffin sectioning technique. Sections (5-μm thick) were rehydrated and stained with H&E, and we observed and photographed the sections with an Olympus AH-2 light microscope (Olympus, Tokyo, Japan). Then, the examination and evaluation were blindly conducted by another experimenter, and five random fields of each section were observed under the microscope. Finally, we photomicrographed and evaluated relevant examination indexes from each section (n = 5).
Immunohistochemistry (IHC) and immunofluorescence (IF)
For IHC analysis, the sections were incubated overnight at 4 °C with anti-StAR (1:1000). IHC analysis was performed using a DAB staining kit to determine the expression level of StAR protein. The intensity of staining was determined by measuring the mean optical density in five random fields for each section (n = 5).
For IF analysis, the sections were incubated with 3β-hydroxysteroid dehydrogenase (3β-HSD) (1:100) overnight at 4 °C. After rewarming for 15 min, the corresponding fluorescent secondary antibody (1:400) was added, and the sections were incubated at room temperature for 1 h in the dark. Nuclear counterstain (DAPI; Sigma-Aldrich) was diluted 1:500 in Tris-buffered saline (TBS) and incubated for 10 min. The number of Leydig cells per unit square of interstitial tissue areas (104 μm2) was calculated by examining 5 randomly selected sites per section (n = 5). All images were captured using an Olympus AH-2 Light Microscope (Olympus, Tokyo, Japan). Analysis of the stained images was performed using Olympus software.
Leydig cell culture and transfection
TM3 Leydig cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cells liberated during digestion were collected and plated at a density of 4 × 105 cells per well in 6-well plates in the medium. According to our experimental data in vivo, the concentration of fetal serum corticosterone in the control group was ~500 ± 49 nM. Thus, we treated the TM3 cells with 500 nM corticosterone for the physiological needs of cells, and 62.5–250 nM corticosterone were low concentrations of corticosterone. MTS assay was conducted to detect the viability of low corticosterone levels on the TM3 cells. The cells were seeded into six-well plates at 4 × 105 cells per well one day before treatment. When the confluence reached about 60%–70%, the cells were transfected with GRα plasmid (3 μg), miR-124-3p mimics (50 nM), Hdac5 siRNA (75 nM) and IGF1 recombinant protein (1 μg/μL) treatment and followed the instructions of Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). After a 72 h treatment, the cells were harvested for subsequent analysis.
Luciferase reporter assay
The segment of the 3′‐untranslated region (3′‐UTR) encompassing the miR-124-3p binding sites was amplified from the Hdac5 gene as the wild type (WT), correspondingly, a mutant 3′‐UTR sequence was obtained using a mature technology, site‐specific in vitro mutation. WT or mutant 3′‐UTR was subsequently inserted downstream of the luciferase gene in the pmirGLO‐control vector (Promega, USA). The Leydig cells were seeded onto 12‐well plates. After culturing for 24 h, co-transfection of miR‐124-3p vector and NC along with a pmirGLO‐control vector carrying WT or mutant 3′‐UTR of Hdac5 into cells were performed using Lipofectamine 3000 reagent according to the manufacturer’s instructions. Then luminescence signals were recorded using the Dual-Luciferase Assay kit (Promega, USA) as indicated in the instructions. All assays were performed in triplicate in a single experiment and repeated six times.
Total RNA extraction, reverse transcription and qRT-PCR
Total RNA was isolated from testicular tissue and TM3 cells using TRIzol Reagent. The total RNA was reverse transcribed using a first strand cDNA synthesis kit. Then, qRT-PCR was performed using an SYBR Green qPCR Master Mix Kit and ABI StepOnePlus cycler (Applied Biosystems, Foster City, CA, USA). The rat primer sequences for the genes used in this study were shown in Table 1. The cycle threshold (Ct) was detected, and the relative expression of genes was determined using the 2△△Ct method with normalization to glyceraldehyde 3-phosphate dehydrogenase for (GAPDH) expression and used as a quantitative control.
The levels of miRNAs were measured by qRT-PCR using miDETECT A Track™ miRNA qRT-PCR kit. The primer of miR-124-3p 5’-CAAGGCACGCGGCGAACGCC-3’ was used. The miRNA expression levels were normalized to the expression of the internal control U6 using the 2△△Ct method.
Chromatin immunoprecipitation-polymerase chain reaction (ChIP-PCR)
The homogenate of testicular tissues or scraped cells was fixed with 1% formaldehyde for 15 min at 37 °C to cross-link DNA and its associated protein. The lysates were then sonicated to shear the DNA to 200–800 bp in length. After sonication, the chromatin solution was incubated with albumin (BSA)-treated protein Gagarose beads with anti-histone 3 lysine 14 (H3K14) or IgG at 4 °C for 16 h, and 1% of the chromatin solution was used as the input. The immunoprecipitated DNA-protein complex that was linked to beads was collected by centrifugation and washed sequentially with some washing buffer. Prepared elution buffer (1% SDS, 0.1 M NaHCO3) was used to elute the DNA-protein complex, and each elution was repeated twice. Samples were incubated overnight at 65 °C with 200 µg/mL proteinase K, and subsequently were purified using a DNA purification kit, following the manufacturer’s protocol. Finally, purified DNA was dissolved with elution buffer finally.
The purified DNA was assayed using qRT-PCR, and the primer sequences are shown in Table 2. With the IgG positive control values subtracted as background (data not shown), the input values were used for quantification and normalized to their corresponding values of immunoprecipitation (IP) samples following the formula (IP/input=2 Ct input DNA – Ct IP DNA).
Western blotting
Testicular protein samples were extracted from offspring rats via RIPA Lysis Buffer (Biyotime, P0013B), and protein concentration was determined using a BCA assay. Protein samples were separated on 12% sodium dodecyl sulfate-polyacrylamide gels and electro-transferred onto polyvinylidene fluoride membrane. Membranes were blocked with 5% BSA for 2 h and then incubated with various antibodies: IGF1 (1:1000), HDAC5 (1:1000), and β-actin (1:5000), respectively. After washing with TBST three times, the membranes were incubated with horseradish peroxidase (HRP) conjugated secondary antibody: anti-rabbit IGF1 and HDAC5, and anti-mouse β-actin. Finally, the protein bands were visualized by using the ECL Plus kit, with the mean optical density representing the relative levels of protein. The experiments were performed in triplicate.
Statistical analysis
Data analysis was performed using SPSS 19 (SPSS Science Inc., Chicago, IL, USA) and Prism 6.0 (Graph Pad Software, La Jolla, CA, USA). Quantitative data are expressed as the mean ± SEM. A two-tailed Student’s t test was used for comparisons between control and treatment groups, and for studies involving more than two groups, data were evaluated with one-way analysis of variance (ANOVA) followed by the Tukey’s post-hoc test. Statistical significance was defined as P < 0.05.
Results
Effects of PDE on testicular histological morphology and testosterone synthesis in offspring rats
To confirm the effects of PDE on the morphology and functional development of testis before and after birth in offspring rats, we observed the testicular morphological alterations and detected the expression of genes related to testosterone synthesis. The results showed that PDE induced the slightly enlarged intratesticular interstitial region in fetal rats, and significantly widened interstitial region and the disordered arrangement of Leydig cells in adult rats (PW12 and PW28) (Fig. 2a); the IF measurement indicated a significant reduction in the number of Leydig cells (3β-HSD+, a specific biomarker of Leydig cells) before and after birth (P < 0.05, P < 0.01, Fig. 2b). Furthermore, the serum testosterone concentrations and intra-testicular testosterone contents were decreased in the PDE offspring before and after birth (P < 0.05, P < 0.01, Fig. 2c, d), the expression levels of steroidal synthases (such as StAR, P450scc, 3β-hsd, 17α-hsd1, and 17β-hsd3) related to testosterone synthesis were decreased in different degrees (P < 0.05, P < 0.01, Fig. 2e), and the protein expression of StAR was reduced persistently (P < 0.05, P < 0.01, Fig. 2f). Above all, PDE can cause abnormal testicular morphology and decreased testosterone synthesis in offspring rats before and after birth.
a Testicular morphology (H&E, 200×, n = 5, red arrow showed the disordered arrangement of Leydig cells); b Leydig cell numbers by IF (white arrow showed the 3β-HSD positive cells stained by red, 400×, n = 5); c, d Testosterone levels by ELISA assay (n = 8); e Testicular steroidogenic genes’ expression by qRT-PCR (n = 8); f StAR protein expression by IHC (yellow arrow showed the StAR positive cells stained by brown, 200×, n = 5). Mean ± SEM. *P < 0.05, **P < 0.01 vs. control. PDE, prenatal dexamethasone exposure. StAR steroidogenic acute regulatory protein, P450scc cytochrome P450 cholesterol side-chain cleavage, 3β-hsd 3β-hydroxysteroid dehydrogenase, 17α-hsd1 17α-hydroxysteroid dehydrogenase 1, 17β-hsd3 17β-hydroxysteroid dehydrogenase 3, GD gestational day, PW postnatal week, IF immunofluorescence, IHC Immunohistochemistry, ELISA enzyme-linked immunoabsorbent assay, qRT-PCR quantitative real-time PCR.
Effects of PDE on serum corticosterone level and the related index regulated-testicular IGF1 epigenetic modification in offspring rats
To explore whether the GC-IGF1 axis is involved in regulating decreased testicular testosterone synthesis, we measured the serum corticosterone concentration and testicular IGF1 expression in offspring rats before and after birth. Compared with the controls, serum corticosterone concentration was significantly decreased in PDE offspring rats on GD20, PW12, and PW28 (P < 0.05, P < 0.01, Fig. 3a), the mRNA and protein expression of testicular IGF1 were both significantly reduced (P < 0.05, P < 0.01, Fig. 3b, c). Furthermore, we explored the epigenetic programming mechanism of IGF1 expression changes caused by endogenous corticosterone at lower than physiological concentrations. We found that the H3K27ac level of testicular Igf1 was decreased in PDE offspring before and after birth (P < 0.05, P < 0.01, Fig. 3d), the mRNA and protein expression of HDAC5 increased continuously (P < 0.05, P < 0.01, Fig. 3e, f). Bioinformatics prediction showed that Hdac5 might be the target gene of miR-124-3p and miR-9, while qRT-PCR verified that only miR-124-3p expression was continuously inhibited (P < 0.05, P < 0.01, Fig. 3g), and miR-9 expression has no significant change (Supplementary Fig. S4). The interesting thing here is there was no significant change in GRα expression in PDE fetal rats, but it was significantly decreased after birth without dexamethasone (P < 0.05, P < 0.01, Fig. 3h). It was suggested that, in the PDE rat model, the serum endogenous corticosterone level and testicular IGF1 expression were continuously decreased before and after birth, showing GC-IGF1 axis consistent change accompanied by the alterations of GRα/miR-124-3p/Hdac5 pathway and Igf1 histone acetylation level (H3K14ac) in testis.
a The serum corticosterone level by ELISA assay (n = 8); b, e, g, h The expression of GRα/miR-124-3p/Hdac5/Igf1/StAR signaling pathway by qRT-PCR (n = 8); c, f The protein expression of IGF1 and HDAC5 by Western blotting (n = 3); d The H3K14ac level of Igf1 by ChIP-PCR (n = 3). Comparisons between groups were performed using Student’s t test, mean ± SEM. *P < 0.05, **P < 0.01 vs. control. Igf1 insulin-like growth factor 1, Hdac5 histone deacetylase 5, H3K14ac histone 3 lysine 14 acetylation, GRα glucocorticoid receptor α, GD gestational day, PW postnatal week, qRT-PCR quantitative real-time PCR.
Effects of chronic stress on serum corticosterone level, testosterone synthesis, and the related index regulated-testicular Igf1 epigenetic modification in offspring rats
We intend to further use adult rats with chronic stress to confirm the GC-IGF1 axis consistent change and its related epigenetic mechanism. The offspring rats were treated with chronic stress (ice-water swimming for 2 weeks at PW10) to increase the serum corticosterone level and then related epigenetic regulating indexes of Igf1 were detected. The results showed that the serum corticosterone concentration in the control group with chronic stress increased by about 1.25 times and that in the PDE group with chronic stress was about 2 times (P < 0.05, P < 0.01, Fig. 4a). Meanwhile, compared with the respective groups without chronic stress, the expression of testicular Igf1 and steroidogenesis-related genes (StAR and 3β-hsd) (P < 0.05, P < 0.01, Fig. 4b, c), intra-testicular testosterone were significantly decreased in the control group with chronic stress but significantly increased in the PDE group with chronic stress (P < 0.05, Fig. 4d); and serum testosterone concentrations were significantly decreased in the control group with chronic stress but has no significant changes in the PDE group with chronic stress (P < 0.05, Fig. 4e). These results indicated that the testicular GC-IGF1 axis of the normal offspring rats showed negative changes, while that of the PDE offspring rats showed positive changes.
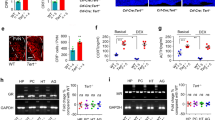
a The serum corticosterone level by ELISA assay (n = 8); b, c, f–h The expression of GRα/miR-124-3p/Hdac5/Igf1/StAR signaling pathway by qRT-PCR (n = 8); d, e Testosterone levels by ELISA assay (n = 8); i The H3K14ac level of Igf1 by ChIP-PCR (n = 3). Comparisons between groups were performed using Student’s t test and ANOVA analysis, Mean ± SEM. *P < 0.05, **P < 0.01 vs. control. StAR steroidogenic acute regulatory protein, P450scc cytochrome P450 cholesterol side-chain cleavage, 3β-hsd 3β-hydroxysteroid dehydrogenase, 17α-hsd1 17α-hydroxysteroid dehydrogenase 1, 17β-hsd3 17β-hydroxysteroid dehydrogenase 3, Igf1 insulin-like growth factor 1, H3K14ac histone 3 lysine 14 acetylation, Hdac5 histone deacetylase 5, GRα glucocorticoid receptor α, qRT-PCR quantitative real-time PCR.
Furthermore, we detected the related index regulated-testicular Igf1 epigenetic modification (Fig. 4f–i). Compared with the respective groups without chronic stress, the testicular GRα expression in control and PDE offspring rats with chronic stress were both increased (P < 0.01); In control groups with chronic stress, miR-124-3p expression showed no significant change, Hdac5 expression was increased and H3K14ac level of Igf1 was decreased (P < 0.01); In PDE groups with chronic stress, miR-124-3p and H3K14ac level of Igf1 were increased, while Hdac5 expression was reduced (P < 0.01). We found that PDE offspring was exposed to low corticosterone after birth, and miR-124-3p/Hdac5/Igf1 signal was inhibited. However, after chronic stress in PDE adult offspring, miR-124-3p/Hdac5/Igf1 signal was activated with the increase of blood corticosterone level. Therefore, we proposed that “GC-IGF1 expression showed consistent changes after birth, and miR-124-3p/Hdac5/Igf1 signal showed corticosterone/GRα-dependent changes”. Taken together, the GC-IGF1 axis showed a consistent change in the PDE adult offspring, and the miR-124-3p/Hdac5/Igf1 histone acetylation exhibited corticosterone/GRα-dependent changing.
Effects of corticosterone on testosterone synthesis and the related index regulated-testicular Igf1 epigenetic modification in TM3 cells
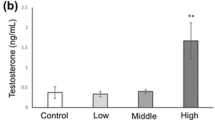
Based on the serum corticosterone level of fetal rats (189.28 ng/mL or 541.06 nM), we used 500 nM corticosterone as the physiological concentration corticosterone. To confirm the effects of corticosterone at lower than physiological concentration on testosterone synthesis function in Leydig cells, we treat TM3 Leydig cells with different concentrations of corticosterone (500, 250, 125, and 62.5 nM). The results verified that corticosterone at lower than physiological concentration (<500 nM) can significantly inhibit testosterone production, StAR and Igf1 expression in Leydig cells (P < 0.05, P < 0.01, Fig. 5a–c). Meanwhile, we also detected the effects of corticosterone at lower than physiological concentration on GRα/miR-124-3p/Hdac5 pathway and Igf1 H3K14ac level. Compared with the physiological concentration group, corticosterone at lower than physiological concentration can induce the decreased GRα and miR-124-3p expression, increased Hdac5 expression, and the decreased H3K14ac of Igf1, the above indexes all showed concentration-dependent changes (P < 0.05, P < 0.01, Fig. 5d–g). It suggested that corticosterone at lower than physiological concentration can regulate testosterone synthesis through GRα/miR-124-3p/Hdac5/Igf1 pathway in Leydig cells.
The TM3 cells were cultured in the presence of corticosterone (62.5–500 nM) for 72 h. a Testosterone concentration in cell culture medium by ELISA assay (n = 6). b–f The expression of GRα/miR-124-3p/Hdac5/Igf1/StAR signaling pathway by qRT-PCR (n = 6). g The H3K14ac level of Igf1 by ChIP-PCR (n = 3). Comparisons between groups were performed using ANOVA analysis, mean ± SEM. *P < 0.05, **P < 0.01 vs. controls. StAR steroidogenic acute regulatory protein, Igf1 insulin-like growth factor 1, GRα glucocorticoid receptor α, Hdac5 histone deacetylase 5, qRT-PCR quantitative real-time PCR.
Corticosterone at lower than physiological concentration inhibits testosterone synthesis through GRα/miR-124-3p/Hdac5/Igf1 pathway
To confirm the GRα/miR-124-3p/Hdac5/Igf1 pathway is involved in the inhibited testosterone synthesis of Leydig cells induced by corticosterone at lower than physiological concentration, we treated TM3 cells with exogenous IGF1, Hdac5 siRNA, miR-124-3p mimics and GRα plasmid. The results showed that exogenous IGF1 supplementation could significantly reverse the inhibitory effect of corticosterone at lower than physiological concentration on StAR expression and testosterone production in Leydig cells (P < 0.05, P < 0.01, Fig. 6a, b). Hdac5 siRNA could increase the H3K14ac and expression levels of Igf1 and StAR expression (P < 0.05, P < 0.01, Fig. 6c–e). Moreover, miR-124-3p mimics significantly reduced the high expression of Hdac5 induced by corticosterone at lower than the physiological concentration (P < 0.01, Fig. 6f). A luciferase reporter assay was used for the analysis of direct regulation of Hdac5 by miR-124-3p. Synchronous transfection of miR-124-3p mimics and WT 3′-UTR of Hdac5 evidently attenuated the luciferase signals, whereas no difference was detected in groups treated with Mut 3′-UTR of Hdac5 or NC vector (P < 0.01, Fig. 6g). Furthermore, we found that GRα plasmid can partly reverse the changes of miR-124-3p, Igf1 and StAR expression induced by corticosterone at lower than physiological concentration. The above results showed that corticosterone at lower than physiological concentration reduced miR-124-3p expression by inhibiting GRα, and targeted to up-regulate Hdac5, then reduced the H3K14ac level and expression of the Igf1, mediating the decrease of StAR expression and testosterone production.
The TM3 cells were cultured in the presence of corticosterone (500 and 62.5 nM) with IGF1 (100 ng/mL), Hdac5 siRNA (20 nM), miR-124-3p mimics (20 nM), and GRα plasmid vector for 72 h. a Testosterone concentration in cell culture medium by ELISA assay; b, e, j StAR mRNA expression by qRT-PCR; f Hdac5 mRNA expression by qRT-PCR; d The H3K14ac level of Igf1 by ChIP-PCR; c, i Igf1 mRNA expression by qRT-PCR; h miR-124-3p expression by qRT-PCR; g The association between miR-124-3p and Hdac5 by luciferase assay. Comparisons between groups were performed using ANOVA, mean ± SEM., n = 6 for all examination indexes. *P < 0.05, **P < 0.01 vs. respective controls. StAR steroidogenic acute regulatory protein, Hdac5 histone deacetylase 5, Igf1 insulin-like growth factor 1, H3K14ac histone 3 lysine 14 acetylation, GRα glucocorticoid receptor α; qRT-PCR quantitative real-time PCR.
Discussion
According to studies, pregnant women with signs of preterm at gestation weeks 24th–34th are treated with dexamethasone to promote lung tissue maturation and reduce other complications, such as respiratory distress syndrome [28, 29]. The classic clinical application of dexamethasone in the treatment of premature infants is an intramuscular injection of 6 mg per 12 h, for a total of 4 times. Combined with the dose conversion between humans and rats (1:6.16) [30], prenatal treatment with dexamethasone in rats at 0.2 mg·kg−1·d−1 in this study is comparable with that used in humans at 0.03 mg·kg−1·d−1, while the standard dose of dexamethasone in clinical treatment during pregnancy is 0.05–0.2 mg·kg−1·d−1[31]. Moreover, ~1/3 treatment of pregnant women changes from preventive to continuous administration due to the difficulty in early diagnosis of preterm delivery and the inefficacy of a single course, resulting in multiple courses of treatment, even more than 11 courses [32, 33]. Besides, testicular morphological development in rats gradually forms from GD9.5, and then enters the masculinization window (GD14-18) and has the testosterone synthesis function [34]. Therefore, (1) to simulate the time of clinical administration of dexamethasone in the middle and late stages of pregnancy for the treatment of threatened preterm labor and (2) the characteristics of testicular development, we subcutaneously administered 0.2 mg·kg−1·d−1 dexamethasone on GD9-20 to establish the PDE rat model. Studies have shown that multiple courses of steroid treatment can affect placental and fetal growth, which may be caused by changes in related factors of maternal blood circulation [35, 36]. It has important theoretical value and clinical significance by using the PDE rat model to explore the short and long-term harm of testicular developmental toxicity and its intrauterine programming mechanism. In this study, dexamethasone was given continuously in the middle and late stages of pregnancy. However, in clinical practice, dexamethasone is given intermittently in the middle and late stages of pregnancy. This research method is somewhat divorced from clinical practice, which to some extent limits the guiding significance of this study conclusions for clinical practice, and this is the deficiency of this study.
It has been reported that PDE can result in low blood cortisol levels in mother marmosets (primates) [37]. Our previous studies also showed that PDE can reduce blood corticosterone concentration in maternal rats [7]. Due to the endogenous glucocorticoids in fetal blood mainly derived from mother [38], it suggested that the decreased fetal blood corticosterone was related to the decreased maternal blood corticosterone in the PDE model. Studies have shown that the adult offspring following prenatal dexamethasone exposure in the maternal spiny mouse have abnormal adrenal reticular zone and reduction of steroid production [39]. Our results also showed that PDE can cause decreased serum corticosterone concentration in puberty (PW12) and adulthood (PW28), which may be associated with adrenal dysplasia in PDE offspring rats [7]. Glucocorticoids at physiological concentrations are known to promote multi-organ maturation, including lungs, heart, and epididymis [40, 41]. Adrenalectomy could induce abnormal testicular morphology and sperm parameters in rats [5]. Therefore, we speculated that corticosterone at lower than the physiological concentration is likely to mediate PDE-induced decreased testosterone synthesis in offspring rats. This study confirms that serum corticosterone concentrations of PDE offspring rats were decreased in different time points (GD20, PW12 and PW28), and the expression levels of testosterone synthesis-related genes (such as StAR, P450scc and 3β-hsd) were decreased, especially the persistent decrease of StAR expression, accompanied by decreased levels of serum testosterone and intra-testicular testosterone. We accidentally found that the serum corticosterone level was increased in the PDE adult offspring rats with chronic stress, the testicular StAR expression, serum and intra-testicular testosterone levels were also increased. In vitro, corticosterone at lower than the physiological concentration can significantly inhibit the StAR expression and testosterone production in Leydig cells. Above all, PDE can induce testicular hypofunctional development in offspring rats mediated by endogenous glucocorticoids (corticosterone) at lower than physiological concentration.
Testicular main endocrine function is testosterone production, which is synthesized in Leydig cells and mainly achieved by the cholesterol transformation through transporters and series of steroidal enzymes, including StAR, P450scc, 3β-hsd, 17β-hsd1, and 17β-hsd3 [42]. Our previous study showed that PDE induced testicular dysplasia mediated by decreased H3K9ac level of StAR in male offspring rats before and after birth [9]. IGF1 is autocrine which is also secreted by Leydig cells, IGF1 and its receptor are involved in the regulation of proliferation, apoptosis and steroid synthesis in multiple testicular cells [43, 44]. Our previous studies have shown that prenatal exposure to xenobiotics (such as caffeine, nicotine, and ethanol) can “negative” regulate the GC-IGF1 axis in multiple organs, including adrenal, bone, and liver, leading to intrauterine “thrifty phenotype” development and early postnatal period “catch-up” compensatory development in offspring rats [2,3,4]. For instance, prenatal caffeine exposure induced the fetal overexposure to maternal corticosterone, resulting in fetal testicular IGF1 expression inhibition and testicular dysplasia. After birth, the serum corticosterone levels were decreased and testicular IGF1 expression were increased, the testosterone synthesis of prenatal caffeine exposure offspring were gradually increased from GD20 to PW12, and even exceeded the control at PW28 (Supplementary Fig. S1). That is, the GC-IGF1 axis “negative” regulate compensatory testicular development [15]. In this study, we interestingly found that serum endogenous corticosterone concentration and testicular IGF1 expression in the PDE offspring rats on GD20-PW28 were persistently decreased; rather, with the serum corticosterone level increased in the adult PDE offspring with chronic stress, the above indicators were increased. In vitro, it was further found that corticosterone at lower physiological concentration could inhibit the Igf1 expression and testosterone production in Leydig cells, and exogenous IGF1 could significantly reverse the inhibited testosterone synthesis. At this point, we proposed that there was a “positive” regulation between blood corticosterone level and testicular IGF1 expression in the PDE rats, which is the “GC-IGF1 axis positive programming”, which mediated the testicular decompensatory development (persistent testicular low-functional development) in the PDE offspring after birth.
It is known that complex epigenetic modification (such as DNA methylation, histone modification, and non-coding RNAs) can cause gene expression changes and then altered multi-organ development programming, that could be reconstructed according to external environment effects [45, 46]. Our previous researches have found that prenatal exposure to xenobiotics (such as dexamethasone, caffeine, and nicotine) induced reprogramming of multi-organ development and homeostasis by changing the histone acetylation of key genes in utero [47,48,49]. For example, prenatal caffeine exposure can inhibit the H3K14ac level of Igf1 and its expression by high corticosterone concentration in utero, thus cause testicular dysplasia [15]. In this study, with the corticosterone concentration increasing in the PDE offspring with chronic stress, the H3K14ac level of testicular Igf1 and its expression were increased, as well as the level of Igf1 and its expression in Leydig cells. Meanwhile, dexamethasone could directly inhibit Igf1 expression in Leydig cells, but it did not change the H3K14ac level of Igf1 (Supplementary Fig. S2). These suggested that dexamethasone may directly inhibit Igf1 expression in Leydig cells in utero, resulting in decreased testosterone synthesis, but such effect cannot be sustained after birth. Above all, corticosterone lower than physiological concentration (rather than dexamethasone) can reduce the H3K14ac level of Igf1 to epigenetic programming its expression before and after birth, resulting in the persistent reduction of testosterone synthesis.
It is known that histone acetyltransferases (HATs) and HDACs jointly regulate histone acetylation modification [50]. We had screened a series of HATs and HDACs expression and found that the expression of testicular Hdac4, Hdac5, Hdac7 and Hdac10 were increased in the PDE fetal rats, but corticosterone at lower than physiological concentration can only increase Hdac5 expression in Leydig cells (Supplementary Fig. S3). Moreover, Hdac5 silencing could significantly reverse the decreased H3K14ac and expression level of Igf1, StAR expression and testosterone production caused by corticosterone at lower physiological concentration. These indicated that corticosterone at lower than physiological concentration induced the decreased H3K14ac and expression levels of Igf1 mediated by increased Hdac5 expression in Leydig cells. Studies have reported that HDACs can be targeted by miRNAs to play their roles. For instance, miR-193b-5p regulates chondrocyte metabolism by directly targeting Hdac7 in interleukin-1β-induced osteoarthritis [51]. Bioinformatics prediction showed that Hdac5 might be the target gene of miR-124-3p and miR-9, our results showed that the expression of miR-124-3p in the fetal testis was significantly inhibited in the PDE group, while the expression of miR-9 was not significantly changed (Supplementary Fig. S4). The expression of miR-124-3p was decreased from PW12 to PW28, while its expression was relatively increased after chronic stress, showing a positive correlation with the level of corticosterone. In TM3 cells, corticosterone at lower than the physiological concentration also significantly reduced the expression of miR-124-3p, and miR-124-3p mimics treatment significantly reversed the promotion effect of low corticosterone on Hdac5 expression, and increase the H3K14ac and expression level of Igf1. Moreover, luciferase reporter gene results showed that synchronous transfection of miR-124-3p mimics and WT 3,-UTR of Hdac5 evidently attenuated the luciferase signals that is miR-124-3p directly target and regulate Hdac5. Above all, corticosterone at lower than physiological concentration up-regulated Hdac5 by inhibiting miR-124-3p, then reduced the H3K14ac level of Igf1 and its expression. In the screening of miRNAs in this study, we only screened the miRNAs targeting the regulation of Hdac5 through the bioinformatics website, without transcriptome sequencing analysis of testicular tissue, which is a deficiency in this study.
Glucocorticoids are known to target the testis by binding directly to GRs [52]. GRs contain two main subunits (GRα and GRβ), GRα is thought to be the main mediator of glucocorticoids action, and gene clusters were regulated by GRα while the presence of endogenous glucocorticoids only [53, 54]. So, we only focus on GRα in this study. We found that there was no significant change of GRα expression in the PDE fetal testis, but it continued to decrease after birth. We hypothesized that on GD20, the fetal testis was affected by both endogenous corticosterone and exogenous dexamethasone, but because they have opposite regulatory effects on the GRα expression, so there was no significant change in the GRα expression in the fetal testis during the intrauterine period. Moreover, the direct effect of dexamethasone on GRα in intrauterine masked the role of endogenous corticosterone at lower concentrations. And there are only endogenous corticosterone at lower than physiological concentration had effects on GRα when the effect of dexamethasone is removed after birth. In this study, we also detected GRβ expression at the same time, and found that the expression of GRβ was decreased at PW12, while the expression of GRβ was not significantly changed at PW28 and GD20 (Supplementary Fig. S5). In Leydig cells, we further found that corticosterone at lower than physiological concentration can inhibit GRα expression. In addition to activating protein-coding genes, GR is also involved in regulating the expression of short non-coding RNAs including miRNAs [55]. We proved that the inhibition of miR-124-3p and StAR expression in Leydig cells by low levels of corticosterone was partially reversed by GRα overexpression. Taken together, corticosterone at lower than physiological concentration reduced miR-124-3p expression by inhibiting GRα, and then miR-124-3p targeted to up-regulate Hdac5, thereby reducing the H3K14ac level of Igf1 and its expression, leading to the decreased StAR expression and testosterone production.
In conclusion, we creatively found that intrauterine glucocorticoids at lower than physiological concentration could reduce testicular IGF1 expression and participate in testicular hypofunctional programming in the PDE offspring, which is “GC-IGF1 axis positive programming” (Fig. 7). In combined with in vivo and in vitro models, we further confirmed that the GRα/miR-124-3p/Hdac5 pathway reduced Igf1 H3K14ac level and its expression, and then inhibited testicular StAR expression and testosterone production. From the perspective of “GC-IGF1 axis positive programming”, this study confirmed the long-term damage of PDE-induced testosterone synthesis in offspring and its intrauterine programming mechanism, providing a novel target and idea for the prevention and treatment of PDE-related fetal-originated testicular dysplasia.
References
Briceno Perez CE, Reyna Villasmil P, Vigil De G. Antenatal corticosteroid therapy: Historical and scientific basis to improve preterm birth management. Eur J Obstet Gynecol Reprod Biol. 2019;234:32–37.
Huang HG, He Z, Zhu CY, Liu L, Kou H, Shen L, et al. Prenatal ethanol exposure-induced adrenal developmental abnormality of male offspring rats and its possible intrauterine programming mechanisms. Toxicol Appl Pharmacol. 2015;288:84–94.
He Z, Zhang JZ, Huang HG, Yuan C, Zhu CY, Magdalou J, et al. Glucocorticoid-activation system mediated glucocorticoid-insulin-like growth factor 1 (GC-IGF1) axis programming alteration of adrenal dysfunction induced by prenatal caffeine exposure. Toxicol Lett. 2019;302:7–17.
Zhou J, Zhu CY, Luo HW, Shen L, Gong J, Wu YM, et al. Two intrauterine programming mechanisms of adult hypercholesterolemia induced by prenatal nicotine exposure in male offspring rats. Faseb J. 2019;33:1110–23.
Silva EJR, Vendramini V, Restelli A, Bertolla RP, Kempinas WG, Avellar MCW. Impact of adrenalectomy and dexamethasone treatment on testicular morphology and sperm parameters in rats: insights into the adrenal control of male reproduction. Andrology. 2014;2:835–46.
Waddell BJ, Bollen M, Wyrwoll CS, Mori TA, Mark PJ. Developmental programming of adult adrenal structure and steroidogenesis: effects of fetal glucocorticoid excess and postnatal dietary omega-3 fatty acids. J Endocrinol. 2010;205:171–8.
Xu D, Chen M, Pan XL, Xia LP, Wang H. Dexamethasone induces fetal developmental toxicity through affecting the placental glucocorticoid barrier and depressing fetal adrenal function. Environ Toxicol Pharmacol. 2011;32:356–63.
Jeje SO, Ola Mudathir FK, Raji Y. Experimental maternal treatment with dexamethasone during lactation induces neonatal testicular and epididymal oxidative stress; Implications for early postnatal exposure. Pathophysiology. 2017;24:261–5.
Liu M, Chen B, Pei LG, Zhang Q, Zou YF, Xiao H, et al. Decreased H3K9ac level of StAR mediated testicular dysplasia induced by prenatal dexamethasone exposure in male offspring rats. Toxicology. 2018;408:1–10.
Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–70.
El Gehani F, Tena Sempere M, Ruskoaho H, Huhtaniemi I. Natriuretic peptides stimulate steroidogenesis in the fetal rat testis. Biol Reprod. 2001;65:595–600.
Sarraj MA, Escalona RM, Umbers A, Kheng Chua H, Small C, Mike G, et al. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (beta glycan) knockout mice. Biol Reprod. 2010;82:153–62.
Wang GM, O’Shaughnessy PJ, Chubb C, Robaire B, Hardy MP. Effects of insulin-like growth factor I on steroidogenic enzyme expression levels in mouse leydig cells. Endocrinology. 2003;144:5058–64.
Liu M, Zhang Q, Pei L, Zou Y, Chen G, Wang H. Corticosterone rather than ethanol epigenetic programmed testicular dysplasia caused by prenatal ethanol exposure in male offspring rats. Epigenetics. 2019;14:245–59.
Pei LG, Zhang Q, Yuan C, Liu M, Zou YF, Lv F, et al. The GC-IGF1 axis-mediated testicular dysplasia caused by prenatal caffeine exposure. J Endocrinol. 2019;242:M17–M32.
Zhang Q, Pei L-G, Liu M, Lv F, Chen G, Wang H. Reduced testicular steroidogenesis in rat offspring by prenatal nicotine exposure: epigenetic programming and heritability via nAChR/HDAC4. Food Chem Toxicol. 2020;135:111057.
Goyal D, Limesand SW, Goyal R. Epigenetic responses and the developmental origins of health and disease. J Endocrinol. 2019;242:T105–T119.
Legoff L, Dali O, D Cruz SC, Suglia A, Gely-Pernot A, Chloé H, et al. Ovarian dysfunction following prenatal exposure to an insecticide, chlordecone, associates with altered epigenetic features. Epigenetics Chromatin. 2019;12:29.
Alamdar A, Tian M, Huang Q, Du X, Zhang J, Liu L, et al. Enhanced histone H3K9 tri-methylation suppresses steroidogenesis in rat testis chronically exposed to arsenic. Ecotoxicol Environ Saf. 2019;170:513–20.
Hong J, Chen F, Wang X, Bai Y, Zhou R, Yingchun L, et al. Exposure of preimplantation embryos to low-dose bisphenol A impairs testes development and suppresses histone acetylation of StAR promoter to reduce production of testosterone in mice. Mol Cell Endocrinol. 2016;427:101–11.
Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11:155–61.
Schober ME, Xingrao K, Bohan X, Block BP, Requena DF, McKnight R, et al. Traumatic brain injury increased IGF-1B mRNA and altered IGF-1 exon 5 and promoter region epigenetic characteristics in the rat pup hippocampus. J Neurotrauma. 2012;29:2075–85.
Yang Q, Sun M, Ramchandran R, J Usha R. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: role of epigenetic regulation. Vasc Pharmacol. 2015;73:20–31.
Gao Y, She R, Wang Q, Li Y, Zhang H. Up-regulation of miR-299 suppressed the invasion and migration of HTR-8/S Vneo trophoblast cells partly via targeting HDAC2 in pre-eclampsia. Biomed Pharmacother. 2018;97:1222–8.
Xing T, Zhu J, Xian J, Li A, Wang X, Wang W, et al. miRNA-548ah promotes the replication and expression of hepatitis B virus by targeting histone deacetylase 4. Life Sci. 2019;219:199–208.
Dong W, Xu D, Hu Z, He X, Guo Z, Jiao Z, et al. Low-functional programming of the CREB/BDNF/TrkB pathway mediates cognitive impairment in male offspring after prenatal dexamethasone exposure. Toxicol Lett. 2017;283:1–12.
Liu L, Liu F, Kou H, Zhang BJ, Xu D, Chen B, et al. Prenatal nicotine exposure induced a hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in intrauterine growth retardation offspring rats. Toxicol Lett. 2012;214:307–13.
Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19.
Roberts D, Brown J, Medley N, Stuart R D. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454.
Reagan Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61.
Miracle X, Di Renzo GC, StARk A, Fanaroff A, Estrany XC, Saling E, et al. Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med. 2008;36:191–6.
Newnham JP, Jobe AH. Should we be prescribing repeated courses of antenatal corticosteroids? Semin Fetal Neonatal Med. 2009;14:157–63.
Elfayomy AK, Almasry SM. Effects of a single course versus repeated courses of antenatal corticosteroids on fetal growth, placental morphometry and the differential regulation of vascular endothelial growth factor. J Obstet Gynaecol Res. 2014;40:2135–45.
Piprek RP. Molecular and cellular machinery of gonadal differentiation in mammals. Int J Dev Biol. 2010;54:779–86.
Zhang C, Xu D, Luo H, Lu J, Liu L, Ping J, et al. Fetal programming of hypothalamus-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. Pt 1
Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79.
Hauser J, Artho AD, Pilloud S, Maier C, Knapman A, Feldon J, et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148:1813–22.
Venihaki M, Carrigan A, Dikkes P, Majzoub JA. Circadian rise in maternal glucocorticoid prevents pulmonary dysplasia in fetal mice with adrenal insufficiency. Proc Natl Acad Sci USA. 2000;97:7336–41.
Quinn TA, Ratnayake U, Melendez MC, Moritz KM, Dickinson H, Walker DW. Adrenal steroidogenesis following prenatal dexamethasone exposure in the spiny mouse. J Endocrinol. 2014;221:347–62.
Au CL, Ngai HK, Yeung CH, Wong PY. Effect of adrenalectomy and hormone replacement on sodium and water transport in the perfused rat cauda epididymidis. J Endocrinol. 1978;77:265–6.
Henriquez A, House J, Miller DB, Snow SJ, Fisher A, Hongzu R, et al. Adrenal-derived stress hormones modulate ozone-induced lung injury and inflammation. Toxicol Appl Pharmacol. 2017;329:249–58.
Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13.
Wen Q, Cheng CY, Liu YX. Development, function and fate of fetal Leydig cells. Semin Cell Dev Biol. 2016;59:89–98.
Yoon MJ, Roser JF. Insulin-like growth factor-I (IGF-I) protects cultured equine Leydig cells from undergoing apoptosis. Anim Reprod Sci. 2010;122:353–8.
de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49. Pt 3
Legault LM, Bertrand Lehouillier V, McGraw S. Pre-implantation alcohol exposure and developmental programming of FASD: an epigenetic perspective. Biochem Cell Biol. 2018;96:117–30.
Xiao H, Wen Y, Pan Z, Shangguan Y, Qin J, Tan Y, et al. Increased H3K27ac level of ACE mediates the intergenerational effect of low peak bone mass induced by prenatal dexamethasone exposure in male offspring rats. Cell Death Dis. 2018;9:638.
Fan G, Zhang Q, Wan Y, Lv F, Chen Y, Ni Y, et al. Decreased levels of H3K9ac and H3K27ac in the promotor region of ovarian P450 aromatase mediated low estradiol synthesis in female offspring rats induced by prenatal nicotine exposure as well as in human granulosa cells after nicotine treatment. Food Chem Toxicol. 2019;128:256–66.
Hu S, Liu K, Luo H, Xu D, Chen L, Zhang L, et al. Caffeine programs hepatic SIRT1-related cholesterol synthesis and hypercholesterolemia via A2AR/cAMP/PKA pathway in adult male offspring rats. Toxicology. 2019;418:11–21.
Egger G, Liang G, Aparicio A, Peter AJ. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63.
Zhang C, Zhang Z, Chang Z, Mao G, Hu S, Zeng A, et al. MiR-193b-5p regulates chondrocytes metabolism by directly targeting histone deacetylase 7 in interleukin-1beta-induced osteoarthritis. J Cell Biochem. 2019;120:12775–84.
Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–25.
Pujols L, Mullol J, Benítez P, Torrego A, Xaubet A, de Haro J, et al. Expression of the glucocorticoid receptor alpha and beta isoforms in human nasal mucosa and polyp epithelial cells. Respir Med. 2003;97:90–6.
Chatzopoulou A, Roy U, Meijer AH, Alia A, Spaink HP, Marcel JMS. Transcriptional and metabolic effects of glucocorticoid receptor alpha and beta signaling in zebrafish. Endocrinology. 2015;156:1757–69.
Tessel MA, Krett NL, Rosen ST. Steroid receptor and microRNA regulation in cancer. Curr Opin Oncol. 2010;22:592–7.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (No. 2020YFA0803900), National Natural Science Foundation of China (Nos. 81673524, 82030111), the Major Technological Innovation Projects of Hubei Province (No. 2019ACA140), Hubei Province’s Outstanding Medical Academic Leader Program, and Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (No. TFJC2018001).
Author information
Authors and Affiliations
Contributions
ML: Conceptualization, Investigation, Writing-Original Draft; YL: Conceptualization, Investigation, Writing-Original Draft; LGP: Investigation; QZ: Supervision; HX: Supervision; YWC: Supervision; HW: Supervision, Project administration, Funding acquisition, Writing - Review and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Liu, M., Liu, Y., Pei, Lg. et al. Prenatal dexamethasone exposure programs the decreased testosterone synthesis in offspring rats by low level of endogenous glucocorticoids. Acta Pharmacol Sin 43, 1461–1472 (2022). https://doi.org/10.1038/s41401-021-00789-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00789-z
Keywords
This article is cited by
-
Dexamethasone exposure during pregnancy triggers metabolic syndrome in offspring via epigenetic alteration of IGF1
Cell Communication and Signaling (2024)
-
Network and mechanism of China’s new energy vehicle industry from the perspective of value chain
Journal of Geographical Sciences (2024)
-
Multi-organ developmental toxicity and its characteristics in fetal mice induced by dexamethasone at different doses, stages, and courses during pregnancy
Archives of Toxicology (2024)