Abstract
The protein levels and activities of calpain-1 and calpain-2 are increased in cardiac mitochondria under pathological conditions including ischemia, diabetes, and sepsis, and transgenic overexpression of mitochondrial-targeted calpain-1 induces dilated heart failure, which underscores an important role of increased calpain in mitochondria in mediating myocardial injury. However, it remains to be determined whether selective inhibition of calpain in mitochondria protects the heart under pathological conditions. In this study, we generated transgenic mice overexpressing mitochondrial-targeted calpastatin in cardiomyocytes. Their hearts were isolated and subjected to global ischemia/reperfusion. Hyperglycemia was induced in the transgenic mice by injections of STZ. We showed that transgenic calpastatin was expressed exclusively in mitochondria isolated from their hearts but not from other organs including skeletal muscle and lung tissues. Transgenic overexpression of mitochondrial-targeted calpastatin significantly attenuated mitochondrial oxidative stress and cell death induced by global ischemia/reperfusion in isolated hearts, and ameliorated mitochondrial oxidative stress, cell death, myocardial remodeling and dysfunction in STZ-treated transgenic mice. The protective effects of mitochondrial-targeted calpastatin were correlated with increased ATP5A1 protein expression and ATP synthase activity in isolated hearts subjected to global ischemia/reperfusion and hearts of STZ-treated transgenic mice. In cultured rat myoblast H9c2 cells, overexpression of mitochondrial-targeted calpastatin maintained the protein levels of ATP5A1 and ATP synthase activity, prevented mitochondrial ROS production and decreased cell death following hypoxia/reoxygenation, whereas upregulation of ATP5A1 or scavenging of mitochondrial ROS by mito-TEMPO abrogated mitochondrial ROS production and decreased cell death. These results confirm the role of calpain in myocardial injury, suggesting that selective inhibition of calpain in myocardial mitochondria by mitochondrial-targeted calpastatin is an effective strategy for alleviating myocardial injury and dysfunction in cardiac pathologies.
Similar content being viewed by others
Introduction
Calpains are a family of calcium-dependent thiol-proteases that participate in a wide variety of biological functions [1]. Among the 15 identified isoforms in the calpain family, calpain-1 and calpain-2 are ubiquitously expressed and have been extensively studied. They are heterodimers that each consists of a distinct large 80-kDa catalytic subunit encoded by the Capn1 and Capn2 gene and a common small 28-kDa regulatory subunit encoded by the Capns1 gene, which is indispensable for calpain-1 and calpain-2 stability and activity. Both calpain-1 and calpain-2 are inhibited by their endogenous inhibitor calpastatin. Calpastatin specifically inhibits calpain-1 and calpain-2 but not other cysteine proteases. Calpain-1 and calpain-2 activities are increased in the diseased heart in various animal models [2,3,4,5,6,7,8,9,10,11,12,13,14] and in patients with myocardial infarction [6] and heart failure [15, 16]. Notably, both pharmacological inhibition of calpain and genetic inhibition of calpain by overexpression of calpastatin or calpain gene knockout have been reported to prevent cardiac apoptosis, reduce myocardial remodeling and improve myocardial function in animal models of various cardiac diseases, including heart failure [17,18,19], myocardial infarction [3, 11, 20,21,22], ischemia/reperfusion (I/R) injury [4, 6, 10, 23,24,25], diabetic cardiomyopathy [12, 26,27,28], septic cardiomyopathy [9, 29], and viral myocarditis [8, 30, 31]. Further evidence supporting the role of calpain in cardiac disease is that cardiac-specific overexpression of calpain-1 is sufficient to cause heart failure in transgenic mice [32]. Thus, calpain may represent a therapeutic target for the treatment of cardiac disease and heart failure.
While it is clear that calpain activation plays an important role in cardiac diseases and heart failure, the underlying mechanisms are incompletely understood, although they appear to be multifactorial. Among the various reported mechanisms, calpain-mediated mitochondrial damage contributes to cardiac disease and heart failure [25, 27, 29, 33]. Early studies demonstrated that calpain induces the release of apoptosis-inducing factor (AIF) in mitochondria through its proteolysis, leading to cardiac apoptosis following I/R [13]. Later, it was reported that calpain activity is increased in mitochondria in the ischemic heart and that this change correlates with complex I defects and myocardial injury [5, 34,35,36]. Pharmacological inhibition of calpain prevents mitochondrial complex I defects and alleviates myocardial injury in the ischemic heart [5, 35]. We recently demonstrated that in diabetes and sepsis, the protein levels of calpain-1 increase in cardiac mitochondria, where calpain-1 cleaves ATP5A1 and consequently disrupts ATP synthase activity, leading to ATP depletion, mitochondrial reactive oxygen species (ROS) generation, and subsequent cardiac cell death [27, 29]. These studies suggest that mitochondrial calpain is involved in cardiac pathology, which is further supported by our most recent finding that overexpression of cardiomyocyte-specific and mitochondrial calpain-1 sufficiently induces dilated heart failure in transgenic mice [33]. These previous studies strongly suggest that targeting mitochondrial calpain may be a useful strategy for alleviating myocardial injury under conditions of oxidative stress. More importantly, evidence from calpain knockout mice and calpastatin overexpressing mice clearly indicates that physiological levels of calpain activity are necessary for a healthy heart [32, 37]. This finding raises concern that global inhibition of calpain may have off-target effects and therefore suggests that precisely targeting calpain in mitochondria may represent a strategy for improving cardiac disease treatment.
In this study, we hypothesized that targeted inhibition of calpain in mitochondria is an effective approach for rescuing ATP synthase activity, reducing mitochondrial ROS production and thereby attenuating myocardial injury under conditions of oxidative stress. To address this hypothesis, we specifically overexpressed mitochondrial calpastatin in cardiomyocytes to selectively inhibit calpain in the mitochondria of cardiomyocytes in vitro and in vivo and determined whether overexpression of mitochondrial calpastatin provides cardioprotection in two different oxidative stress-related conditions: I/R and hyperglycemia.
Materials and methods
Animals
This research conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication, 8th Edition, 2011). All experimental protocols were approved by the Animal Use Subcommittee of Soochow University, China. Breeding pairs of C57BL/6 mice were purchased from the Cyagen Biosciences (Suzhou, China).
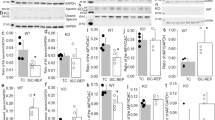
A transgenic vector containing full-length mouse Calpastatin cDNA (coding region) with mitochondrial targeting signal peptide (MTS) and myc-tag under the tetracycline transactivator (tTA)-inducible mouse alpha-myosin heavy chain (α-MHC) promoter was constructed (Fig. 1a), and transgenic mice containing the mouse α-MHC promoter along with the MTS-Calpastatin-myc-tag cDNA (Tg-mtCAST) were generated as previously described [33]. The Tg-mtCAST mice were then crossed with transgenic mice with cardiomyocyte-specific overexpression of tTA (Tg-tTA) to produce wild-type, Tg-tTA, Tg-mtCAST and Tg-mtCAST/tTA mice. The presence of the Calpastatin and tTA transgenes was determined by polymerase chain reaction (PCR) of DNA from mouse tail tips (Supplementary Fig. S1). The beta-2 microglobulin (B2M) gene was amplified as a loading control. The sequences of the primers for the Calpastatin and tTA transgenes and B2M were as follows. Calpastatin: 5′-GTTGGCTTAGGCTGCTTTTCGT-3′ (forward), 5′-CCAGACTCCGTGACACCCCTT-3′ (reverse); tTA: 5′-AGCGCATTAGAGCTGCTTAATGAGGTC-3′ (forward), 5′-GTCGTAATAATGGCGGCATACTATC-3′ (reverse); B2M: 5′-CAGACTCTGCGATGTTTCCA-3′ (forward), 5′-GCCTGAGCACTTCCAGAAAC-3′ (reverse). Only double transgenic Tg-mtCAST/tTA mice expressed mitochondrial calpastatin in cardiomyocytes. Both Tg-tTA mice and the transgenic vector containing the tTA-inducible mouse α-MHC promoter were kindly provided by Dr. Jeffrey Robbins [38].
a Schematic structure of the transgene. The transgene was released from the vector by digestion with BamH1. hGH: human growth hormone. b A representative Western blot showing transgenic calpastatin (CAST), GAPDH and VDAC1 expression in mitochondria and cytosol in heart tissues from Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates. c–e Adult Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates were injected with streptozotocin (STZ, 50 mg· kg−1· d−1, i.p.) for five consecutive days. Two months after STZ injection, myocardial function was assessed by echocardiography, and necrotic cell death was determined by Evans blue dye (EBD) staining. c Fractional shortening (%). d E/A ratio. e Upper panel: representative microphotographs of Evans blue staining (red) and nuclear staining (blue). Lower panel: quantitation of the percentage of Evans blue-positive area relative to total area (%). The data are presented as the mean ± SD of six different heart tissues from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+STZ.
All animals were housed in a temperature- and humidity-controlled facility on a 12-h light and dark cycle with water and food ad libitum.
Hyperglycemic mice
Hyperglycemia was induced in adult mice (male, 2 months old) by intraperitoneal injections of streptozotocin (STZ, 50 mg· kg−1· d−1, Sigma, Oakville, ON, Canada) for five consecutive days. Seventy-two hours after the last injection of STZ, whole blood was obtained from the mouse tail vein, and random glucose levels were measured using the OneTouch Ultra 2 blood glucose monitoring system (LifeScan, Inc., Malvern, PA, USA). The mice were considered hyperglycemic and used for the study only if their blood glucose levels were ≥15 mmol/L 72 h after STZ injection, whereas citrate buffer-injected mice were used as normoglycemic controls (blood glucose < 12 mmol/L) [12, 26,27,28].
Global I/R in isolated hearts
Experiments were performed using isolated isovolumic heart preparations as we described previously [24]. Briefly, whole hearts were isolated from adult male mice (2 months old) and perfused on the Langendorff system (Radnoti LLC, Covina, CA, USA). After an equilibration period of 20 min, the isolated whole hearts were subjected to 45 min of global ischemia by stopping perfusion (zero-flow) followed by reperfusion for 30 min. The perfusate temperature was maintained at 37 °C throughout the protocol (before ischemia and during ischemia and reperfusion).
Hypoxia/reoxygenation (H/R) in H9c2 cells
Rat myoblast H9c2 cells were purchased from the American Type Culture Collection (ATCC, Gaithersburg, MD, USA), and cultured H9c2 cells were employed within 10 passages. H9c2 cells were subjected to H/R as we described previously [10]. In brief, H9c2 cells were seeded in a 24-well culture plate. For the induction of hypoxia, we placed 24-well culture plates in a sealed bag containing the GENbag anaera system (bioMérieux Canada, Inc., Saint-Laurent, QC, Canada) for 24 h at 37 °C. Hypoxia was monitored using an anaera indicator (bioMérieux Canada, Inc.). The GENbag anaera system reduced the O2 concentration by 50% in the chamber within 30 min, and after 3 h, the O2 concentration was below 0.1%. Reoxygenation was achieved by replacing the culture medium with fresh medium and returning the culture plates to a normal CO2 incubator (37 °C, 5% CO2) for another 24 h.
Echocardiography
Mice were anesthetized with inhaled isoflurane (1%) and imaged using a 40 MHz linear array transducer attached to a preclinical ultrasound system (Vevo 2100, FUJIFILM Visual Sonics, Toronto, ON, Canada) with a nominal in-plane spatial resolution of 40 μm (axial) × 80 μm (lateral). M-mode and 2-D parasternal short-axis scans (133 frames/s) at the level of the papillary muscles were employed to assess changes in left ventricular (LV) end-systolic inner diameter, LV end-diastolic inner diameter, LV posterior wall thickness in end-diastole and end-systole, and fractional shortening (FS). To assess diastolic function, pulsed wave Doppler measurements of maximal early (E) and late (A) transmittal velocities in diastole were obtained in the apical view with a cursor at mitral valve inflow.
Histological analysis
Heart tissues were fixed in 4% paraformaldehyde (Sigma) at 4 °C for 48 h and then routinely processed, wax-embedded and sectioned. After processing, the tissue sections (5 μm thick) were stained with fluorescence dye-conjugated wheat germ agglutinin (WGA, Thermo Fisher Scientific, Waltham, MA, USA) to detect the cell membranes (red) and Hoechst 33342 (Thermo Fisher Scientific) to detect nuclei (blue) or with a saturated solution of picric acid containing 1% Sirius red (Sigma) for collagen deposition measurement [12]. The signals were visualized by fluorescence or light microscopy and photographed, and the cardiomyocyte cross-sectional area and collagen content were determined by computer-assisted morphometry (Image-Pro Plus Version 6.0, Media Cybernetics, Inc., Rockville, MD, USA). For each sample, at least 30 fields were analyzed.
Cell membrane permeability to Evans blue dye (EBD)
EBD (Sigma) was dissolved in saline and injected into mice (100 mg/kg body weight, i.p.). Twenty-four hours later, the mice were euthanized by cervical dislocation under anesthesia with a mixture of ketamine (100 mg/kg)/xylazine (5 mg/kg, i.p., Sigma) after ensuring that they did not respond to a needle prick of the skin. The hearts were harvested, embedded in optimal cutting temperature (OCT) compound (Sakura, VWR International, Mississauga, ON, Canada), snap frozen in liquid nitrogen, and cut into 5-μm cryosections. EBD uptake (red) was visualized under a fluorescence microscope. EBD-positive cells were counted in whole sections and the data were presented as a percentage of EBD-positive area to total area.
Determination of oxidative stress
The formation of H2O2 in heart tissue lysates and cell lysates was assessed using the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Thermo Fisher Scientific). Oxidative damage was determined by measuring malondialdehyde (MDA) and protein carbonyl contents in heart tissues using a TBARS assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) and Protein Carbonyl Colorimetric Assay Kit (Cayman Chemical Company), respectively. All these experiments were conducted following the manufacturer’s instructions.
Measurement of H2O2 production in freshly isolated mitochondria
Mitochondria were isolated from freshly harvested hearts as described previously [39]. Mitochondrial H2O2 production was measured by the addition of pyruvate/malate or succinate using an Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Adenoviral infection
H9c2 cells were infected with an adenoviral vector containing mitochondrial calpastatin (Ad-mtCAST, Vector Biolabs, Malvern, PA, USA) or ATP5A1 (Ad-ATP5A1, SignaGen, Rockville, MD, USA) at a multiplicity of infection of 100 PFU/cell. An adenoviral vector containing beta-gal (Ad-gal, Vector Biolabs) served as a control [27]. Adenovirus-mediated gene transfer was performed as previously described [40].
ATP synthase activity
ATP synthase activity in heart tissue lysates was measured using an assay coupled with pyruvate kinase, which converts ADP to ATP and produces pyruvate from phosphoenolpyruvate, as previously described [29, 41].
Measurement of mitochondrial superoxide generation
Mitochondrial superoxide generation in live H9c2 cells was assessed using MitoSOXTM Red Mitochondrial Superoxide Indicator (Thermo Fisher Scientific) following the manufacturer’s instructions [42].
Western blot analysis
Heart tissues or H9c2 cells were homogenized in lysis buffer and subjected to SDS-polyacrylamide gel electrophoresis. After the proteins were transferred to PVDF membranes, Western blot analysis was conducted to determine the protein levels of interest using specific antibodies. Primary antibodies against calpastatin (catalog number: 4146, 1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA), CAPN1 (catalog number: 2556, 1:1000 dilution, Cell Signaling Technology), CAPN2 (catalog number: 2539, 1:1000 dilution, Cell Signaling Technology), ATP5A1 (catalog number: 459240, 1:1000 dilution, Invitrogen Canada Inc., Burlington, ON, Canada), VDAC1 (catalog number: 4866, 1:1000 dilution, Cell Signaling Technology), phosphorylated and total extracellular signal-regulated protein kinase ½ (ERK1/2, catalog number: 9101 for phosphorylated ERK1/2 and catalog number: 9102 for total ERK1/2, 1:1000 dilution, Cell Signaling Technology), and GAPDH (catalog number: 10494-1-AP, 1:1000 dilution, Proteintech, Chicago, IL, USA) were employed as previously described [27, 40, 42]. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (H + L) secondary antibody was purchased from BOSTER Biological Technology (catalog number: BA1051, Wuhan, China), and HRP-conjugated goat anti-rabbit IgG (H + L) was purchased from Jackson ImmunoResearch (catalog number: 136080, West Grove, PA, USA).
Real-time reverse transcription PCR
Total RNA was extracted from heart tissues using TRIzol Reagent (Sigma) following the manufacturer’s instructions. Real-time reverse transcription PCR was performed to analyze the mRNA levels of ANP, collagen-III and GAPDH as previously described [12].
Active caspase-3
As described previously [28, 43], caspase-3 activity in heart tissues was measured using a Caspase-3 Fluorescence Assay Kit (Biomol Research Laboratories, Inc., Plymouth, PA, USA). This assay measures the fluorescence intensity of the chromophore AMC cleaved from the C terminus of the peptide substrate. The fluorescence intensity of cleaved AMC was quantified with a multilabel reader (excitation, 355 nm; emission, 460 nm; Victor 3; Wallac, PerkinElmer, Waltham, MA, USA) and normalized to the intensity of cleaved AMC in the inhibitor-treated samples as a control.
Determination of cellular DNA fragmentation
Cultured H9c2 cells were prelabeled with BrdU (1 μg/mL). DNA fragmentation was evaluated using a Cellular DNA Fragmentation ELISA Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer’s instructions.
Measurement of lactate dehydrogenase (LDH)
The levels of LDH in culture medium were measured using a commercially available kit (Sigma) following the manufacturer’s instructions.
Cell viability
Cell Counting Kit-8 (CCK-8, Sigma) was used to determine the number of viable H9c2 cells following the manufacturer’s instructions.
Statistical analysis
The data are expressed as the mean ± SD. Student’s t test was employed to compare data between two groups. ANOVA followed by the Newman-Keuls test was performed for multigroup comparisons. A P value of less than 0.05 was considered significant.
Results
Transgenic overexpression of mitochondrial calpastatin prevents cardiac cell death and myocardial dysfunction in hyperglycemic mice
Our recent study demonstrated that overexpression of mitochondrial calpastatin prevents apoptosis of cultured cardiomyocytes induced by high glucose [27]. To determine whether mitochondrial calpastatin prevents adverse myocardial changes during hyperglycemia in vivo, we generated transgenic mice expressing mitochondrial calpastatin specifically in cardiomyocytes (Tg-mtCAST/tTA mice). The Tg-mtCAST/tTA mice bred well and grew normally into adulthood without exhibiting obvious health problems. In these mice, transgenic calpastatin was expressed exclusively in mitochondria in the heart (Fig. 1b) but not in other organs, including skeletal muscle (Supplementary Fig. S2a) or the lung (Supplementary Fig. S2b). Tg-mtCAST, Tg-tTA and wild-type mice did not express transgenic calpastatin in the heart and thus served as wild-type controls for the following studies.
Tg-mtCAST/tTA mice and their wild-type littermates were rendered hyperglycemic by STZ. Two months after STZ injection, mice exhibited hyperglycemia. Tg-mtCAST/tTA mice and their wild-type littermates displayed similar blood glucose levels and body weights under normoglycemic and hyperglycemic conditions (Supplementary Table S1), suggesting that transgenic cardiomyocyte-specific overexpression of mitochondrial calpastatin did not affect systemic metabolism. Echocardiographic analysis showed no difference of myocardial function between Tg-mtCAST/tTA mice and their wild-type littermates under normoglycemic conditions (Supplementary Table S1). Consistent with our previous studies [12, 26, 27, 42], myocardial dysfunction was observed in hyperglycemic mice, as indicated by a lower EF% and FS% in these mice than in normoglycemic wild-type mice. However, myocardial function was relatively preserved in hyperglycemic Tg-mtCAST/tTA mice (Fig. 1c, d, Supplementary Table S1). The Evans blue staining assay revealed a greater increase in cell membrane permeability in the hearts of hyperglycemic wild-type mice, which was indicative of cell death (Fig. 1e), than in those of hyperglycemic Tg-mtCAST/tTA mice. These results indicate that mitochondrial calpastatin reduces myocardial cell death and prevents myocardial dysfunction in hyperglycemic mice.
Overexpression of mitochondrial calpastatin attenuates myocardial hypertrophy and fibrosis in hyperglycemic mice
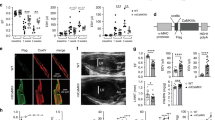
Histological analysis of the cardiomyocyte cross-sectional area in wild-type mice showed that cardiomyocyte size was increased in hearts of hyperglycemic mice compared to those of normoglycemic mice, indicating cardiomyocyte hypertrophy. In contrast, cardiomyocyte size was not increased in the hearts of hyperglycemic Tg-mtCAST/tTA mice compared to those of normoglycemic mice (Fig. 2a, b). Additionally, gene expression analysis revealed upregulation of ANP mRNA in the hearts of hyperglycemic wild-type mice compared to those of normoglycemic mice; however, the levels of ANP mRNA were much lower in the hearts of hyperglycemic Tg-mtCAST/tTA mice than in those of hyperglycemic WT mice (Fig. 2c). Under normoglycemic conditions, there were no differences between Tg-mtCAST/tTA mice and wild-type littermates in terms of cardiomyocyte size or ANP mRNA levels. These results suggest that mitochondrial calpastatin alleviates myocardial hypertrophy in hyperglycemic mice.
Adult Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates were injected with STZ (50 mg· kg−1 ·d−1, i.p.) for five consecutive days. Two months after STZ injection, heart tissues were collected and processed for histological analyses. a A representative histological picture of fluorescence-conjugated wheat germ agglutinin staining (red). b Quantitation of the cardiomyocyte cross-sectional area. c Real-time RT-PCR analysis of ANP mRNA expression in heart tissues. d A representative histological picture of Sirius red staining (red). e Quantitation of collagen deposition. f Real-time RT-PCR analysis of collagen-III mRNA expression in heart tissues. The data are presented as the mean ± SD of six different heart tissues from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+STZ.
Myocardial fibrosis is another characteristic change that occurs in the chronic hyperglycemic heart. Histological analysis of myocardial collagen deposition revealed an increase in fibrosis in the hearts of hyperglycemic wild-type mice compared to those of normoglycemic mice, which was accompanied by upregulation of collagen-III mRNA expression. However, overexpression of mitochondrial calpastatin correlated with significant reductions in collagen deposition (Fig. 2d, e) and collagen-III mRNA expression (Fig. 2f) in the hearts of hyperglycemic Tg-mtCAST/tTA mice. Transgenic overexpression of mitochondrial-targeted calpastatin did not affect myocardial fibrosis in the hearts of normoglycemic Tg-mtCAST/tTA mice or wild-type mice.
Overexpression of mitochondrial calpastatin alleviates cardiac injury induced by I/R
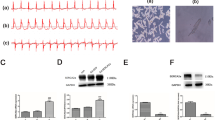
Mitochondrial calpain activity is increased in the ischemic heart [5, 34,35,36]. In line with these previous reports, we showed that CAPN1 protein levels were also elevated in mitochondria in the heart following global I/R, whereas CAPN2 protein levels remained unchanged (Fig. 3a and Supplementary Fig. S3a and S3b). Upregulation of the CAPN1 protein in mitochondria correlated with myocardial cell death, as evidenced by an increase in the percentage of Evans blue-positive area in the wild-type mouse heart. However, the percentage of Evans blue-positive cells was much lower in the hearts of Tg-mtCAST/tTA mice than in those of wild-type mice following global I/R (Fig. 3b). Similarly, H/R increased the protein levels of CAPN1 but not CAPN2 in the mitochondria of H9c2 cells (Fig. 3c and Supplementary Fig. S3c and S3d). Expression of mitochondrial calpastatin attenuated caspase-3 activity (Fig. 3d), DNA fragmentation (Fig. 3e) and LDH release (Fig. 3f) induced by H/R in H9c2 cells. Thus, mitochondrial calpastatin improved cell viability in response to H/R (Fig. 3g). These results demonstrate that targeted inhibition of mitochondrial calpain protects cardiomyocytes against I/R-induced injury.
a, b Hearts isolated from adult Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates were subjected to 45 min of global ischemia and 30 min of reperfusion. a Heart tissues were collected, and mitochondria were isolated. Representative Western blots for CAPN1, CAPN2, VDAC1, and GAPDH from two out of the five hearts are shown. b Necrotic cell death was determined by Evans blue dye staining. The upper panel is a representative microphotograph of Evans blue staining (red) and nuclear staining (blue), and the bottom panel is the quantitation of the percentage of the Evans blue-positive area relative to the total area (%). The data are presented as the mean ± SD of five different hearts from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+I/R. c–g H9c2 cells were infected with Ad-mtCAST or Ad-gal for 24 h and then subjected to 24 h of hypoxia followed by 24 h of reoxygenation (H/R). H9c2 cells were collected, and mitochondria were isolated. c A representative Western blot for CAPN1, CAPN2, VDAC1, and GAPDH from the three experiments. Apoptosis was determined based on caspase-3 activity (d) and DNA fragmentation (e). f The LDH level in the culture medium was measured. g Cell viability was determined. The data are the mean ± SD from three different experiments. *P < 0.05 vs Ad-gal + sham and #P < 0.05 vs Ad-gal + H/R.
Mitochondrial calpastatin prevents the reduction in ATP5A1 protein expression and ATP synthase activity as well as mitochondrial oxidative stress induced by hyperglycemia and I/R
We recently reported that calpain-1 in mitochondria targets and cleaves ATP5A1, thereby impairing ATP synthase activity in cardiomyocytes under hyperglycemic conditions [27]. Consistently, we showed that the proteins levels of ATP5A1 and ATP synthase activity were much lower in the hearts of hyperglycemic wild-type mice than in those of normoglycemic wild-type mice (Fig. 4a, b). Transgenic overexpression of mitochondrial calpastatin preserved the protein levels of ATP5A1 and ATP synthase activity in the hearts of hyperglycemic mice (Fig. 4a, b). Similarly, global I/R decreased the protein levels of ATP5A1 and ATP synthase activity in the hearts of wild-type mice (Fig. 4c, d). However, the protein levels of ATP5A1 and ATP synthase activity were much higher in the hearts of Tg-mtCAST/tTA mice than in those of wild-type mice following global I/R (Fig. 4c, d). To provide direct evidence for the protective effects of mitochondrial calpastatin on ATP synthase, we overexpressed mitochondrial calpastatin in H9c2 cells and then exposed these cells to H/R. As shown in Fig. 4e, f, H/R resulted in downregulation of ATP5A1 protein levels and a reduction in ATP synthase activity, both of which were normalized by overexpression of mitochondrial calpastatin. These results suggest that targeted inhibition of mitochondrial calpain may protect mitochondrial ATP synthase activity against the effect of hyperglycemia or I/R.
a, b Adult Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates were injected with STZ (50 mg ·kg−1 ·d−1, i.p.) for five consecutive days. Two months after STZ injection, heart tissues were collected. a The upper panel shows representative Western blots for ATP5A1 and GAPDH from two out of the six hearts, and the bottom panel shows the quantification of the ATP5A1/GAPDH ratio for all hearts. b ATP synthase activity was measured. The data are presented as the mean ± SD of six different heart tissues from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+STZ. c, d Hearts isolated from adult TG and WT mice were subjected to 45 min of global (zero-flow) ischemia and 30 min of reperfusion. c Upper panel: representative Western blots for ATP5A1 and GAPDH from two out of the five hearts. Lower panel: quantification of the ATP5A1/GAPDH ratio for all hearts. d ATP synthase activity was measured. The data are presented as the mean ± SD of five different heart tissues from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+I/R. e, f H9c2 cells were infected with Ad-mtCAST or Ad-gal for 24 h and then subjected to 24 h of hypoxia followed by 24 h of reoxygenation (H/R). e Upper panel: a representative Western blot for ATP5A1 and VDAC1 from three different experiments. Lower panel: quantitation of the ATP5A1/VDAC1 ratio. f ATP synthase activity was measured. The data are the mean ± SD of three different experiments. *P < 0.05 vs Ad-gal + sham and #P < 0.05 vs Ad-gal + H/R.
Impairment of mitochondrial ATP synthase or mitochondrial dysfunction induces excessive ROS production and subsequent oxidative damage, which further exacerbates mitochondrial dysfunction and damage. Therefore, we evaluated mitochondrial oxidative stress. In the hearts of hyperglycemic mice, the production of mitochondrial H2O2 (Fig. 5a, b) and MDA (Fig. 5c), a lipid peroxidation marker, was increased in the hearts of wild-type mice compared to those of Tg-mtCAST/tTA mice. Transgenic mitochondrial calpastatin prevented the hyperglycemia-induced activation of the ROS-sensitive ERK1/2 signaling pathway in the mouse heart (Fig. 5d). In isolated wild-type hearts, global I/R induced increases in mitochondrial H2O2 production, MDA production and the content of protein carbonyl, a protein oxidation marker. However, the levels of mitochondrial H2O2 (Fig. 6a), MDA production (Fig. 6b) and protein carbonyl content (Fig. 6c) were much lower in the hearts of Tg-mtCAST/tTA mice than in those of wild-type mice following global I/R. Similarly, H/R increased mitochondrial ROS production in H9c2 cells, as determined by MitoSOXTM Red staining (Fig. 6d) and H2O2 production (Fig. 6e), which was prevented by infection with an adenoviral vector expressing mitochondrial calpastatin (Ad-mtCAST). Taken together, these results suggest that mitochondrial calpastatin prevents mitochondrial ROS production and oxidative damage induced by hyperglycemia and I/R.
Adult Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates were injected with STZ (50 mg· kg−1 ·d−1, i.p.) for five consecutive days. Two months after STZ injection, heart tissues were collected, and mitochondria were isolated. Mitochondrial ROS production was measured with pyruvate/malate (a) or succinate (b) as substrates. The level of the lipid peroxidation marker MDA was evaluated (c). d Upper panel: representative Western blots for p-ERK1/2, ERK1/2 and GAPDH from two or four out of the six hearts. Lower panel: quantification of the p-ERK1/2/GAPDH ratio for all hearts. The data are presented as the mean ± SD of six different heart tissues from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+STZ.
Hearts isolated from adult Tg-mtCAST/tTA (TG) mice and their wild-type (WT) littermates were subjected to 45 min of global (zero-flow) ischemia and 30 min of reperfusion. The levels of mitochondrial H2O2 (a) and MDA (b) and the protein carbonyl content (c) were determined. The data are presented as the mean ± SD of five different heart tissues from each group. *P < 0.05 vs WT+sham and #P < 0.05 vs WT+I/R. d, e H9c2 cells were infected with Ad-mtCAST or Ad-gal for 24 h and then subjected to 24 h of hypoxia followed by 24 h of reoxygenation (H/R). d Representative MitoSOXTM Red staining from three different experiments. e Quantification of mitochondrial H2O2 production. The data are the mean ± SD of three different experiments. *P < 0.05 vs Ad-gal + sham and #P < 0.05 vs Ad-gal + H/R.
Overexpression of ATP5A1 reduces mitochondrial ROS production and cardiomyocyte death induced by H/R
We then determined whether overexpression of ATP5A1 reduces mitochondrial ROS production and prevents cell death in cardiomyocytes following H/R. As shown in Fig. 7a, b, H/R induced an increase in mitochondrial ROS production in H9c2 cells, which was attenuated by infection with an adenoviral vector expressing ATP5A1. Overexpression of ATP5A1 reduced apoptosis as determined by caspase-3 activity (Fig. 7c) and DNA fragmentation (Fig. 7d), inhibited LDH release (Fig. 7e) and improved the viability (Fig. 7f) of H9c2 cells following H/R. Thus, overexpression of ATP5A1 reduces mitochondrial ROS production and cardiomyocyte death induced by H/R.
H9c2 cells were infected with Ad-ATP5A1 or Ad-gal for 24 h and then subjected to 24 h of hypoxia followed by 24 h of reoxygenation (H/R). a Representative MitoSOXTM Red staining from three different experiments. b Quantitation of mitochondrial H2O2 production. c, d Apoptosis was determined based on caspase-3 activity (c) and DNA fragmentation (d). e The level of LDH in the culture medium was measured. f Cell viability was determined. The data are the mean ± SD of three different experiments. *P < 0.05 vs Ad-gal + sham and #P < 0.05 vs Ad-gal + H/R.
Selective inhibition of mitochondrial ROS alleviates cardiomyocyte injury induced by H/R
To determine the role of mitochondrial ROS in I/R-induced injury, we incubated H9c2 cells with the mitochondrial selective ROS scavenger mito-TEMPO or vehicle and then exposed the cells to H/R. Incubation with mito-TEMPO reduced mitochondrial ROS production induced by H/R in H9c2 cells (Fig. 8a, b). H/R-induced LDH release (Fig. 8c) and apoptosis, as determined by caspase-3 activity (Fig. 8d) and DNA fragmentation (Fig. 8e), were attenuated by mito-TEMPO. Consequently, the viability of H9c2 cells was decreased following H/R, and this effect of H/R was attenuated by mito-TEMPO (Fig. 8f). Selective inhibition of mitochondrial ROS alleviates cardiomyocyte injury induced by H/R.
H9c2 cells were incubated with mito-TEMPO or vehicle and then subjected to 24 h of hypoxia followed by 24 h of reoxygenation. a Representative MitoSOXTM Red staining from three different experiments. b Quantitation of mitochondrial H2O2 production. c The level of LDH in the culture medium was measured. d, e Apoptosis was determined based on caspase-3 activity (d) and DNA fragmentation (e). f Cell viability was measured. The data are the mean ± SD from three different experiments. *P < 0.05 vs vehicle + sham and #P < 0.05 vs vehicle + H/R.
Discussion
We and others have reported elevations in calpain protein and activity in mitochondria in the heart under various pathological conditions [5, 27, 29, 34,35,36], and it has been found that transgenic overexpression of mitochondrial-targeted calpain-1 induces dilated heart failure in mice [33]. We extended our study to investigate whether selective inhibition of calpain in mitochondria alleviates myocardial injury induced by oxidative stress and found that inhibition of mitochondrial calpain by mitochondrial-targeted calpastatin provides cardioprotection against I/R- and hyperglycemia-induced injury. We demonstrated that mitochondrial-targeted calpastatin preserves ATP5A1 protein levels and ATP synthase activity, thereby preventing ROS production and oxidative stress and leading to cardioprotection under hyperglycemic conditions or following I/R. We suggest that increased calpain expression in mitochondria leads to activation of the downstream ROS-sensitive ERK1/2 signaling pathway, which is implicated in cardiac pathology.
Hyperglycemia exacerbates cardiac mitochondrial oxidative damage and ROS-sensitive ERK1/2 signaling and induces myocardial cell death and remodeling in mice, which is consistent with our recent report [42]. Transgenic overexpression of mitochondrial calpastatin significantly attenuated hyperglycemia-induced mitochondrial oxidative stress, reduced ERK1/2 signaling and protected the hearts of hyperglycemic mice. Similarly, overexpression of mitochondrial calpastatin reduced I/R- or H/R-induced mitochondrial oxidative stress in isolated hearts and H9c2 cells. Since inhibition of mitochondrial ROS has been shown to improve cardiac remodeling and preserve cardiac function in heart failure [44, 45], the attenuation of hyperglycemia or ischemia-induced mitochondrial oxidative damage may be one of the downstream mechanisms contributing to the protection conferred by mitochondrial calpastatin. Furthermore, mitochondrial ROS can lead to cell death, and targeted inhibition of mitochondrial ROS may represent a potential therapeutic approach for cardiac disease [42, 44]. Our results confirmed that mito-TEMPO prevented cell death, an effect that was concomitant with the cardioprotection conferred by overexpression of mitochondrial calpastatin in the hearts of hyperglycemic mice and in the heart following global I/R. One potential downstream mechanism may be linked to ERK1/2 signaling, as our previous study showed that inhibition of ERK1/2 reduces apoptosis of cardiomyocytes induced by high glucose [42]. It is well known that calpastatin specifically inhibits calpain-1 and calpain-2. Thus, our data further support an important role for mitochondrial calpain in mitochondrial ROS production and cardiac cell death induced by hyperglycemia and I/R, conditions under which calpain-1 and calpain-2 are increased in myocardial mitochondria [5, 27, 34,35,36].
Attenuation but not complete inhibition of hyperglycemia- or I/R-induced cardiac injury conferred by overexpression of mitochondrial calpastatin suggests that calpain plays a role in other organelles. Indeed, this is supported by several lines of evidence. First, global overexpression of calpastatin alleviates diabetic, ischemic and septic cardiomyopathy, viral myocarditis and heart failure in animal models [8, 12, 14, 19, 24]. Second, deletion of Capns1 in cardiomyocytes prevents diabetic, ischemic and septic cardiomyopathy [11, 12, 27, 29]. Third, transgenic overexpression of calpain-1 sufficiently induces dilated heart failure [32]. The underlying mechanisms of this phenomenon have been proposed to be associated with NF-κB signaling [11, 46], protein kinase C-α activation [47, 48], proteolysis of cell structural proteins [49, 50], etc. Taken together, our study suggests that calpain is a potential therapeutic target for alleviating cardiac injury and protecting myocardial function.
Our recent studies demonstrated that increased calpain-1 in mitochondria targets and cleaves ATP5A1 and subsequently compromises ATP synthase activity in septic and diabetic cardiomyopathy [27, 29]. In line with these previous findings, overexpression of mitochondrial calpastatin preserved ATP5A1 protein levels and ATP synthase activity in cultured cardiomyocytes and the heart in response to hyperglycemia, I/R or H/R. Consistent with our findings, a recent study showed that pharmacological inhibition of mitochondrial calpain normalizes lipopolysaccharide-induced cleavage of ATP5A1 and myocardial dysfunction in mice [51]. The impairment of ATP synthase activity resulting from calpain-mediated cleavage of ATP5A1 results in ATP depletion, which directly contributes to myocardial dysfunction. On the other hand, disruption of ATP synthase results in excess electron “backup” in individual electron transfer complexes, in particular complexes I and III, from which escape of electrons is a principal source of ROS. ROS-mediated oxidative damage to mitochondrial electron transfer complexes may lead to a vicious cycle of excessive mitochondrial ROS production, oxidative damage and mitochondrial dysfunction, events that lead to cell death. Loss of cardiomyocytes and resultant cardiac remodeling are the primary pathological bases for myocardial dysfunction and heart failure. Indeed, we showed that overexpression of ATP5A1 rescued ATP synthase activity and subsequently attenuated mitochondrial ROS production and cell death in H9c2 cells following H/R, which is consistent with our previous reports in cardiomyocytes under hyperglycemic and septic conditions [27, 29]. It is worthwhile to mention that overexpression of ATP5A1 attenuated but did not abrogate mitochondrial ROS production and cell death induced by hyperglycemia, I/R or H/R, suggesting that additional mechanisms may be involved in mitochondrial calpain-mediated ROS production. In fact, other calpain targets in mitochondria have been reported. For example, calpain has also been shown to target and disrupt electron transfer complex I within mitochondria in the heart following global I/R, and disruption of complex I contributes to mitochondrial ROS production and cell death [5]. AIF is another target of calpain in mitochondria, and its release in mitochondria after cleavage by calpain induces cardiac apoptosis following I/R [13].
Given that physiological levels of calpain activity may be necessary for a healthy heart, studies have shown that transgenic overexpression of calpastatin results in the development of degenerative cardiomyopathy [32] and that deletion of calpain-2 is embryonic lethal [37]. In addition, we recently reported that calpain-2 is protective against doxorubicin-induced cardiotoxicity [52]. This evidence strongly suggests that global inhibition of calpain within cardiomyocytes may not be the best strategy and underscores the necessity of precisely targeting calpain in organelles for cardiac diseases. Taken together, our results provide a rationale for targeting calpain inhibitors to mitochondria and demonstrate that targeted inhibition of calpain in mitochondria is beneficial for alleviating myocardial injury and preventing myocardial dysfunction in cardiac pathologies.
References
Goll DE, Thompson VF, Li H, Wei W, Cong J. The Calpain system. Physiol Rev. 2003;83:731–801.
Chen Q, Thompson J, Hu Y, Lesnefsky EJ. Cardiomyocyte specific deletion of p53 decreases cell injury during ischemia-reperfusion: role of mitochondria. Free Radic Biol Med. 2020;158:162–70.
Chen K, He L, Li Y, Li X, Qiu C, Pei H, et al. Inhibition of GPR35 preserves mitochondrial function after myocardial infarction by targeting calpain 1/2. J Cardiovasc Pharmacol. 2020;75:556–63.
Penela P, Inserte J, Ramos P, Rodriguez-Sinovas A, Garcia-Dorado D, Mayor F Jr. Degradation of GRK2 and AKT is an early and detrimental event in myocardial ischemia/reperfusion. EBioMedicine. 2019;48:605–18.
Chen Q, Thompson J, Hu Y, Dean J, Lesnefsky EJ. Inhibition of the ubiquitous calpains protects complex I activity and enables improved mitophagy in the heart following ischemia-reperfusion. Am J Physiol Cell Physiol. 2019;317:C910–C21.
Barefield DY, McNamara JW, Lynch TL, Kuster DWD, Govindan S, Haar L, et al. Ablation of the calpain-targeted site in cardiac myosin binding protein-C is cardioprotective during ischemia-reperfusion injury. J Mol Cell Cardiol. 2019;129:236–46.
Aluja D, Inserte J, Penela P, Ramos P, Ribas C, Iniguez MA, et al. Calpains mediate isoproterenol-induced hypertrophy through modulation of GRK2. Basic Res Cardiol. 2019;114:121.
Li M, Su Y, Yu Y, Yu Y, Wang X, Zou Y, et al. Dual roles of calpain in facilitating Coxsackievirus B3 replication and prompting inflammation in acute myocarditis. Int J Cardiol. 2016;221:1123–31.
Freitas ACS, Figueiredo MJ, Campos EC, Soave DF, Ramos SG, Tanowitz HB, et al. Activation of both the calpain and ubiquitin-proteasome systems contributes to septic cardiomyopathy through dystrophin loss/disruption and mtor inhibition. PLoS ONE. 2016;11:e0166839.
Zheng D, Wang G, Li S, Fan G-C, Peng T. Calpain-1 induces endoplasmic reticulum stress in promoting cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim Biophys Acta Mol Basis Dis. 2015;1852:882–92.
Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, et al. Deficiency of Capn4 gene inhibits nuclear factor-κb (NF-κB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem. 2012;287:27480–9.
Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, et al. targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–94.
Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, et al. Activation of mitochondrial μ-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun. 2011;415:533–8.
Li X, Li Y, Shan L, Shen E, Chen R, Peng T. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res. 2009;83:72–9.
Yang D, Ma S, Tan Y, Li D, Tang B, Zhang X, et al. Increased expression of calpain and elevated activity of calcineurin in the myocardium of patients with congestive heart failure. Int J Mol Med. 2010;26:159–64.
Wang JC, Zhao Y, Li XD, Zhou NN, Sun H, Sun YY. Proteolysis by endogenous calpain I leads to the activation of calcineurin in human heart. Clin Lab. 2012;58:1145–52.
Ahmad HA, Lu L, Ye S, Schwartz GG, Greyson CR. Calpain inhibition preserves talin and attenuates right heart failure in acute pulmonary hypertension. Am J Respir Cell Mol Biol. 2012;47:379–86.
Wu CY, Chen B, Jiang YP, Jia Z, Martin DW, Liu S, et al. Calpain-dependent cleavage of junctophilin-2 and T-tubule remodeling in a mouse model of reversible heart failure. J Am Heart Assoc. 2014;3:e000527.
Wang Y, Chen B, Huang CK, Guo A, Wu J, Zhang X, et al. Targeting calpain for heart failure therapy: implications from multiple murine models. JACC Basic Transl Sci. 2018;3:503–17.
Poncelas M, Inserte J, Aluja D, Hernando V, Vilardosa U, Garcia-Dorado D. Delayed, oral pharmacological inhibition of calpains attenuates adverse post-infarction remodelling. Cardiovasc Res. 2017;113:950–61.
Kudo-Sakamoto Y, Akazawa H, Ito K, Takano J, Yano M, Yabumoto C, et al. Calpain-dependent cleavage of n-cadherin is involved in the progression of post-myocardial infarction remodeling. J Biol Chem. 2014;289:19408–19.
Mani SK, Balasubramanian S, Zavadzkas JA, Jeffords LB, Rivers WT, Zile MR, et al. Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1744–H1751.
Lu HT, Feng RQ, Tang JK, Zhou JJ, Gao F, Ren J. CaMKII/calpain interaction mediates ischemia/reperfusion injury in isolated rat hearts. Cell Death Dis. 2020;11:388.
Shan L, Li J, Wei M, Ma J, Wan L, Zhu W, et al. Disruption of Rac1 signaling reduces ischemia–reperfusion injury in the diabetic heart by inhibiting calpain. Free Radic Biol Med. 2010;49:1804–14.
Guan L, Che Z, Meng X, Yu Y, Li M, Yu Z, et al. MCU up-regulation contributes to myocardial ischemia-reperfusion injury through calpain/opa-1–mediated mitochondrial fusion/mitophagy inhibition. J Cell Mol Med. 2019;23:7830–43.
Teng X, Ji C, Zhong H, Zheng D, Ni R, Hill DJ, et al. Selective deletion of endothelial cell calpain in mice reduces diabetic cardiomyopathy by improving angiogenesis. Diabetologia. 2019;62:860–72.
Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, et al. Mitochondrial calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: a novel mechanism contributing to diabetic cardiomyopathy. Diabetes. 2016;65:255–68.
Li Y, Li Y, Feng Q, Arnold M, Peng T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res. 2009;84:100–10.
Ni R, Zheng D, Wang Q, Yu Y, Chen R, Sun T, et al. Deletion of Capn4 protects the heart against endotoxemic injury by preventing ATP synthase disruption and inhibiting mitochondrial superoxide generation. Circ Heart Fail. 2015;8:988–96.
Meng Y, Sun T, Wu C, Dong C, Xiong S. Calpain regulates CVB3 induced viral myocarditis by promoting autophagic flux upon infection. Microbes Infect. 2020;22:46–54.
Yu Y, Shi H, Yu Y, Liu M, Li M, Liu X, et al. Inhibition of calpain alleviates coxsackievirus B3-induced myocarditis through suppressing the canonical NLRP3 inflammasome/caspase-1-mediated and noncanonical caspase-11-mediated pyroptosis pathways. Am J Transl Res. 2020;12:1954–64.
Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, et al. Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–8.
Cao T, Fan S, Zheng D, Wang G, Yu Y, Chen R, et al. Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res Cardiol. 2019;114:117.
Thompson J, Hu Y, Lesnefsky EJ, Chen Q. Activation of mitochondrial calpain and increased cardiac injury: beyond AIF release. Am J Physiol Heart Circ Physiol. 2016;310:H376–H384.
Shintani-Ishida K, Yoshida K. Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia-reperfusion. Int J Cardiol. 2015;197:26–32.
Chen Q, Lesnefsky EJ. Heart mitochondria and calpain 1: Location, function, and targets. Biochim Biophys Acta Mol Basis Dis. 2015;1852:2372–8.
Dutt P, Croall DE, Arthur JSC, Veyra TD, Williams K, Elce JS, et al. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:1–11.
Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–16.
Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–9.
Li Y, Arnold JMO, Pampillo M, Babwah AV, Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med. 2009;46:51–61.
Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, et al. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol. 2010;299:H529–H540.
Ni R, Cao T, Xiong S, Ma J, Fan G-C, Lacefield JC, et al. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12–23.
Hu H, Li X, Li Y, Wang L, Mehta S, Feng Q, et al. Calpain-1 induces apoptosis in pulmonary microvascular endothelial cells under septic conditions. Microvasc Res. 2009;78:33–9.
Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, et al. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82.
Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–46.
Han Q, Liu Q, Zhang H, Lu M, Wang H, Tang F, et al. Simvastatin improves cardiac hypertrophy in diabetic rats by attenuation of oxidative stress and inflammation induced by calpain-1-mediated activation of nuclear factor-κb (NF-κB). Med Sci Monit. 2019;25:1232–41.
Yoshida KI, Hirata T, Akita Y, Mizukami Y, Yamaguchi K, Sorimachi Y, et al. Translocation of protein kinase C-α, δ and ϵ isoforms in ischemic rat heart. Biochim Biophys Acta Mol Basis Dis. 1996;1317:36–44.
Zhang Y, Matkovich SJ, Duan X, Diwan A, Kang MY, Dorn GW 2nd. Receptor-independent protein kinase C alpha (PKCalpha) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J Biol Chem. 2011;286:26943–51.
Guo A, Wang Y, Chen B, Wang Y, Yuan J, Zhang L, et al. E-C coupling structural protein junctophilin-2 encodes a stress-adaptive transcription regulator. Science. 2018;362:eaan3303.
Papp Z, van der Velden J, Stienen GJM. Calpain-I induced alterations in the cytoskeletal structure and impaired mechanical properties of single myocytes of rat heart. Cardiovasc Res. 2000;45:981–93.
Koentges C, Cimolai MC, Pfeil K, Wolf D, Marchini T, Tarkhnishvili A, et al. Impaired SIRT3 activity mediates cardiac dysfunction in endotoxemia by calpain-dependent disruption of ATP synthesis. J Mol Cell Cardiol. 2019;133:138–47.
Zheng D, Su Z, Zhang Y, Ni R, Fan G-C, Robbins J, et al. Calpain-2 promotes MKP-1 expression protecting cardiomyocytes in both in vitro and in vivo mouse models of doxorubicin-induced cardiotoxicity. Arch Toxicol. 2019;93:1051–65.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province (BK20171216) and Priority Academic Program Development of Jiangsu Higher Education Institutions. The funding bodies played no role in the design of the study, the collection, analysis, or interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
DZ, TC, GCF, JQ, and TQP designed the research; DZ, TC, and LLZ performed the research; DZ, TC, LLZ and JQ analyzed the data; DZ and TQP wrote the paper; GCF, JQ, and TQP discussed and revised the paper. All authors approved the present form of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, D., Cao, T., Zhang, Ll. et al. Targeted inhibition of calpain in mitochondria alleviates oxidative stress-induced myocardial injury. Acta Pharmacol Sin 42, 909–920 (2021). https://doi.org/10.1038/s41401-020-00526-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-00526-y
Keywords
This article is cited by
-
Nicotinamide mononucleotide as a therapeutic agent to alleviate multi-organ failure in sepsis
Journal of Translational Medicine (2023)
-
Landscape of molecular crosstalk between SARS-CoV-2 infection and cardiovascular diseases: emphasis on mitochondrial dysfunction and immune-inflammation
Journal of Translational Medicine (2023)
-
Calpain-mediated protein targets in cardiac mitochondria following ischemia–reperfusion
Scientific Reports (2022)
-
Inhibition of calpain reduces cell apoptosis by suppressing mitochondrial fission in acute viral myocarditis
Cell Biology and Toxicology (2022)