Abstract
Objective
To explore the role of p300 in the context of paclitaxel (PTX) resistance in triple-negative breast cancer (TNBC) cells, focusing on its interaction with the phosphoenolpyruvate carboxykinase 1 (PCK1)/adenosine monophosphate-activated protein kinase (AMPK) pathway.
Methods
The expression of p300 and PCK1 at the messenger ribonucleic acid (mRNA) level was detected using a quantitative polymerase chain reaction. The GeneCards and GEPIA databases were used to investigate the relationship between p300 and PCK1. The MDA-MB-231/PTX cell line, known for its PTX resistance, was chosen to understand the specific role of p300 in such cells. The Lipofectamine™ 3000 reagent was used to transfer the p300 small interfering RNA and the overexpression of PCK1 plasmid into MDA-MB-231/PTX. The expression levels of p300, PCK1, 5′AMPK and phosphorylated AMPK (p-AMPK) were determined using the western blot test.
Results
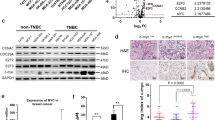
In TNBC cancer tissue, the expression of p300 was increased compared with TNBC paracancerous tissue (P < 0.05). In the MDA-MB-231 cell line of TNBC, the expression of p300 was lower than in the PTX-resistant TNBC cells (MDA-MB-231/PTX) (P < 0.05). The PCK1 expression was decreased in the TNBC cancer tissue compared with TNBC paracancerous tissue, and the PCK1 expression was reduced in MDA-MB-231/PTX than in MDA-MB-231 (P < 0.05) indicating that PCK1 was involved in the resistance function. Additionally, p-AMPK was decreased in MDA-MB-231/PTX compared with MDA-MB-231 (P < 0.05). The adenosine triphosphate (ATP) level was also detected and was significantly lower in MDA-MB-231/PTX than in MDA-MB-231 (P < 0.05). Additionally, cell proliferation increased significantly in MDA-MB-231/PTX at 48 and 72 h (P < 0.05) suggesting that MDA-MB-231/PTX cells obtained the resistance function which was associated with AMPK and ATP level. When p300 was inhibited, p-AMPK and ATP levels elevated in MDA-MB-231/PTX (P < 0.05). When PCK1 was suppressed, the ATP consumption rate decreased, and cell proliferation increased (P < 0.05). However, there were no changes in p300.
Conclusions
In MDA-MB-231/PTX, p300 can inhibit p-AMPK and ATP levels by inhibiting PCK1 expression. Our findings suggest that targeting p300 could modulate the PCK1/AMPK axis, offering a potential therapeutic avenue for overcoming PTX resistance in TNBC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
References
Newton EE, Mueller LE, Treadwell SM, Morris CA, Machado HL. Molecular targets of triple-negative breast cancer: where do we stand? Cancers. 2022;14:482 https://doi.org/10.3390/cancers14030482
Li Y, Zhan Z, Yin X, Fu S, Deng X. Targeted therapeutic strategies for triple-negative breast cancer. Front Oncol. 2021;11:731535 https://doi.org/10.3389/fonc.2021.731535
Baranova A, Krasnoselskyi M, Starikov V, Kartashov S, Zhulkevych I, Vlasenkoet V, et al. Triple-negative breast cancer: current treatment strategies and factors of negative prognosis. J Med Life. 2022;15:153–61. https://doi.org/10.25122/jml-2021-0108
de Boniface J, Frisell J, Kühn T, Wiklander-Bråkenhielm I, Dembrower K, Nyman P, et al. False-negative rate in the extended prospective TATTOO trial evaluating targeted axillary dissection by carbon tattooing in clinically node-positive breast cancer patients receiving neoadjuvant systemic therapy. Breast Cancer Res Treat. 2022;193:589–95. https://doi.org/10.1007/s10549-022-06588-2
Umeh-Garcia M, O’Geen H, Simion C, Gephart MH, Segal DJ, Sweeney CA. Aberrant promoter methylation contributes to LRIG1 silencing in basal/triple-negative breast cancer. Br J Cancer. 2022;127:436–48. https://doi.org/10.1038/s41416-022-01812-8
Liao M, Qin R, Huang W, Zhu HP, Peng F, Han B, et al. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J Hematol Oncol. 2022;15:44 https://doi.org/10.1186/s13045-022-01260-0
Xiu M, Zhang P, Wang X, Fan Y, Li Q, Li Q, et al. Survival outcomes for dose-dense paclitaxel plus carboplatin neoadjuvant vs standard adjuvant chemotherapy in stage II to III triple-negative breast cancer: a prospective cohort study with propensity-matched analysis. Int J Cancer. 2022;151:578–89. https://doi.org/10.1002/ijc.34022
Asaduzzaman M, Constantinou S, Min H, Gallon J, Lin ML, Singh P, et al. Tumour suppressor EP300, a modulator of paclitaxel resistance and stemness, is downregulated in metaplastic breast cancer [published correction appears in Breast Cancer Res Treat. 2018 Jan 5]. Breast Cancer Res Treat. 2017;163:461–74. https://doi.org/10.1007/s10549-017-4202-z
Rao X, Tang P, Li Y, Fu GX, Chen SZ, Xu XY, et al. CBP/P300 inhibitors mitigate radiation-induced GI Syndrome by promoting intestinal stem cell-mediated crypt regeneration. Int J Radiat Oncol Biol Phys. 2021;110:1210–21. https://doi.org/10.1016/j.ijrobp.2021.01.046
Qin X, Chen H, Tu L, Ma Y, Liu N, Zhang HW, et al. Potent inhibition of HIF1α and p300 interaction by a constrained peptide derived from CITED2. J Med Chem. 2021;64:13693–703. https://doi.org/10.1021/acs.jmedchem.1c01043
Mukheem Mudabbir MA, Mundlamuri RC, Mariyappa N, Kumar RA, Velmurugan J, Bhargava GB, et al. P300 in mesial temporal lobe epilepsy and its correlation with cognition—a MEG based prospective case-control study. Epilepsy Behav. 2021;114:107619 https://doi.org/10.1016/j.yebeh.2020.107619
Mastracchio A, Lai C, Digiammarino E, Ready DB, Lasko LM, Bromberg KD, et al. Discovery of a potent and selective covalent p300/CBP inhibitor. ACS Med Chem Lett. 2021;12:726–31. https://doi.org/10.1021/acsmedchemlett.0c00654
Lim CK, Efthymios M, Tan W, Autio MI, Tiang Z, Li PY, et al. Dimethyl sulfoxide (DMSO) enhances direct cardiac reprogramming by inhibiting the bromodomain of coactivators CBP/p300. J Mol Cell Cardiol. 2021;160:15–26. https://doi.org/10.1016/j.yjmcc.2021.06.008
Li TY, Sleiman MB, Li H, Gao AW, Mottis A, Bachmann AM, et al. The transcriptional coactivator CBP/p300 is an evolutionarily conserved node that promotes longevity in response to mitochondrial stress. Nat Aging. 2021;1:165–78. https://doi.org/10.1038/s43587-020-00025-z
Spriano F, Gaudio E, Cascione L, Tarantelli C, Melle F, Motta G, et al. Antitumor activity of the dual BET and CBP/EP300 inhibitor NEO2734. Blood Adv. 2020;4:4124–35. https://doi.org/10.1182/bloodadvances.2020001879
Ferrazoli N, Donadon C, Rezende A, Skarzynski PH, Sanfins MD. The application of P300-long-latency auditory-evoked potential in Parkinson disease. Int Arch Otorhinolaryngol. 2022;26:e158–e166. https://doi.org/10.1055/s-0040-1722250
Luyckx I, Bolar N, Diness BR, Hove HB, Verstraeten A, Loeys BL. Aortic aneurysm: an underestimated serious finding in the EP300 mutation phenotypical spectrum. Eur J Med Genet. 2019;62:96 https://doi.org/10.1016/j.ejmg.2018.06.008
Akil A, Ezzikouri S, El Feydi AE, Benazzouz M, Afifi R, Diagne AG, et al. Associations of genetic variants in the transcriptional coactivators EP300 and PCAF with hepatocellular carcinoma. Cancer Epidemiol. 2012;36:e300–5. https://doi.org/10.1016/j.canep.2012.05.011
Alsamri H, Hasasna HE, Baby B, Alneyadi A, Dhaheri YA, Ayoub MA, et al. Carnosol Is a novel inhibitor of p300 acetyltransferase in breast cancer. Front Oncol. 2021;11:664403 https://doi.org/10.3389/fonc.2021.664403
Gutiérrez-Salmerón M, García-Martínez JM, Martínez-Useros J, Fernández-Aceñero MJ, Viollet B, Olivier S, et al. Paradoxical activation of AMPK by glucose drives selective EP300 activity in colorectal cancer. PLoS Biol. 2020;18:e3000732 https://doi.org/10.1371/journal.pbio.3000732
Tuo L, Xiang J, Pan X, Hu JL, Tang H, Liang L, et al. PCK1 negatively regulates cell cycle progression and hepatoma cell proliferation via the AMPK/p27Kip1 axis. J Exp Clin Cancer Res. 2019;38:50 https://doi.org/10.1186/s13046-019-1029-y
Park SY, Chung YS, Park SY, Kim SH. Role of AMPK in regulation of oxaliplatin-resistant human colorectal cancer. Biomedicines. 2022;10:2690 https://doi.org/10.3390/biomedicines10112690
Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. 2023;24:255–72. https://doi.org/10.1038/s41580-022-00547-x
ElBaset MA, Salem RS, Ayman F, Ayman N, Shaban N, Afifi SM, et al. Effect of empagliflozin on thioacetamide-induced liver injury in rats: role of AMPK/SIRT-1/HIF-1α pathway in halting liver fibrosis. Antioxidants. 2022;11:2152 https://doi.org/10.3390/antiox11112152
Khamaru P, Chakraborty S, Bhattacharyya A. AMPK activator AICAR in combination with anti-mouse IL10 mAb restores the functionality of intra-tumoral Tfh cells in the 4T1 mouse model. Cell Immunol. 2022;382:104639 https://doi.org/10.1016/j.cellimm.2022.104639
Chen LM, Yang PP, Al Haq AT, Hwang PA, Lai YC, Weng YS, et al. Oligo-Fucoidan supplementation enhances the effect of Olaparib on preventing metastasis and recurrence of triple-negative breast cancer in mice. J Biomed Sci. 2022;29:70 https://doi.org/10.1186/s12929-022-00855-6
Wei C, Zou H, Xiao T, Liu XY, Wang QQ, Cheng JL, et al. TQFL12, a novel synthetic derivative of TQ, inhibits triple-negative breast cancer metastasis and invasion through activating AMPK/ACC pathway. J Cell Mol Med. 2021;25:10101–10. https://doi.org/10.1111/jcmm.16945
Zhao T, Zhang T, Zhang Y, Zhou B, Lu X. Paclitaxel resistance modulated by the interaction between TRPS1 and AF178030.2 in triple-negative breast cancer. Evid Based Complement Altern Med. 2022;2022:6019975 https://doi.org/10.1155/2022/6019975
Padilla J, Lee BS, Zhai K, Rentz B, Bobo T, Dowling NM, et al. A heme-binding transcription factor BACH1 regulates lactate catabolism suggesting a combined therapy for triple-negative breast cancer. Cells. 2022;11:1177 https://doi.org/10.3390/cells11071177
Welti J, Sharp A, Brooks N, Yuan W, McNair C, Chand SN, et al. Targeting the p300/CBP axis in lethal prostate cancer. Cancer Discov. 2021;11:1118–37. https://doi.org/10.1158/2159-8290.CD-20-0751
Liu R, Yang H, Chen Z, Zhou KX, Shi QY, Li JY, et al. Design, synthesis and biological evaluation of (R)-5-methylpyrrolidin-2-ones as p300 bromodomain inhibitors with anti-tumor activities in multiple tumor lines. Bioorg Chem. 2022;124:105803 https://doi.org/10.1016/j.bioorg.2022.105803
Li S, Jin J, Daly I, Liu C, Cichocki A. Feature selection method based on Menger curvature and LDA theory for a P300 brain-computer interface. J Neural Eng. 2021; https://doi.org/10.1088/1741-2552/ac42b4
Liu MX, Jin L, Sun SJ, Liu P, Feng X, Cheng ZL, et al. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene. 2018;37:1637–53. https://doi.org/10.1038/s41388-017-0070-6
Tuo L, Xiang J, Pan X, Gao QZ, Zhang GJ, Yang Y, et al. PCK1 downregulation promotes TXNRD1 expression and hepatoma cell growth via the Nrf2/Keap1 pathway. Front Oncol. 2018;8:611 https://doi.org/10.3389/fonc.2018.00611. Published 2018 Dec 17
Tang Y, Zhang Y, Wang C, Sun ZY, Li LF, Cheng SQ, et al. Overexpression of PCK1 gene antagonizes hepatocellular carcinoma through the activation of gluconeogenesis and suppression of glycolysis pathways. Cell Physiol Biochem. 2018;47:344–55. https://doi.org/10.1159/000489811
Shao F, Bian X, Wang J, Xu DQ, Guo W, Jiang HF, et al. Prognostic Impact of PCK1 protein kinase activity-dependent nuclear SREBP1 activation in non-small-cell lung carcinoma. Front Oncol. 2021;11:561247 https://doi.org/10.3389/fonc.2021.561247
Yamaguchi N, Weinberg EM, Nguyen A, Liberti MV, Goodarzi H, Janjigian YY, et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. Elife. 2019;8:e52135 https://doi.org/10.7554/eLife.52135
Sánchez-Mir L, Soto T, Franco A, Madrid M, Viana RA, Vicente J, et al. Rho1 GTPase and PKC ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS One. 2014;9:e88020 https://doi.org/10.1371/journal.pone.0088020
Ge Y, Zhang Y, Li R, Chen W, Li Y, Chen G. Berberine regulated Gck, G6pc, Pck1 and Srebp-1c expression and activated AMP-activated protein kinase in primary rat hepatocytes. Int J Biol Sci. 2011;7:673–84. https://doi.org/10.7150/ijbs.7.673
Xu D, Wang Z, Xia Y, Shao F, Xia WY, Wei YK, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530–5. https://doi.org/10.1038/s41586-020-2183-2
Mercado JJ, Gancedo JM. Regulatory regions in the yeast FBP1 and PCK1 genes. FEBS Lett. 1992;311:110–4. https://doi.org/10.1016/0014-5793(92)81379-z
Shi H, Fang R, Li Y, Li LL, Zhang WY, Wang HW, et al. The oncoprotein HBXIP suppresses gluconeogenesis through modulating PCK1 to enhance the growth of hepatoma cells. Cancer Lett. 2016;382:147–56. https://doi.org/10.1016/j.canlet.2016.08.025
Blenman KRM, Marczyk M, Karn T, Qing T, Li XT, Gunasekharan V, et al. Predictive markers of response to neoadjuvant durvalumab with nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide in basal-like triple-negative breast cancer. Clin Cancer Res. 2022;28:2587–97. https://doi.org/10.1158/1078-0432.CCR-21-3215
Li J, Xu X, Peng X. NDC80 enhances cisplatin-resistance in triple-negative breast cancer [published correction appears in Arch Med Res. 2023;54(5):102833]. Arch Med Res. 2022;53:378–87. https://doi.org/10.1016/j.arcmed.2022.03.003
Chen Y, Feng X, Yuan Y, Jiang J, Zhang P, Zhang B. Identification of a novel mechanism for reversal of doxorubicin-induced chemotherapy resistance by TXNIP in triple-negative breast cancer via promoting reactive oxygen-mediated DNA damage. Cell Death Dis. 2022;13:338 https://doi.org/10.1038/s41419-022-04783-z
Nath A, Mitra S, Mistry T, Pal R, Nasare VD. Molecular targets and therapeutics in chemoresistance of triple-negative breast cancer. Med Oncol. 2021;39:14 https://doi.org/10.1007/s12032-021-01610-x
Yu G, Chen W, Li X, Yu L, Xu XY, Ruan Q, et al. TWIST1-EP300 expedites gastric cancer cell resistance to apatinib by activating the expression of COL1A2. Anal Cell Pathol. 2022;2022:5374262 https://doi.org/10.1155/2022/5374262
Jiang S, Luo Y, Zhan Z, Tang ZM, Zou JR, Ying Y, et al. Amp-activated protein kinase re-sensitizes A549 to paclitaxel via up-regulating solute carrier organic anion transporter family member 1b3 expression. Cell Signal. 2022;91:110215 https://doi.org/10.1016/j.cellsig.2021.110215
Wu CH, Liu FC, Pan CH, Lai MT, Lan SJ, Wu CH, et al. Suppression of cell growth, migration and drug resistance by ethanolic extract of antrodia cinnamomea in human lung cancer A549 cells and C57bl/6j allograft tumor model. Int J Mol Sci. 2018;19:791 https://doi.org/10.3390/ijms19030791
Sereni MI, Baldelli E, Gambara G, Ravaggi A, Hodge KA, Alberts DS, et al. Kinase-driven metabolic signalling as a predictor of response to carboplatin-paclitaxel adjuvant treatment in advanced ovarian cancers. Br J Cancer. 2017;117:494–502. https://doi.org/10.1038/bjc.2017.195
Funding
National Natural Science Foundation of China (grant no. 82060530).
Author information
Authors and Affiliations
Contributions
Peng-Wei Zhao: Performed experimental work, data collection or management, data analysis, and manuscript writing/editing. Jia-Xian Cui: Data collection or management, data analysis, manuscript writing/editing. Xiu-Mei Wang: Conceived and designed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and participation consent
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Inner Mongolia Medical University (NO. YDK2020021025), and informed consent was obtained from all participants. All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent for publication All authors final approval of the version to be published.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, PW., Cui, JX. & Wang, XM. Upregulation of p300 in paclitaxel-resistant TNBC: implications for cell proliferation via the PCK1/AMPK axis. Pharmacogenomics J 24, 5 (2024). https://doi.org/10.1038/s41397-024-00324-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00324-3


