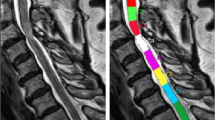
Abstract
Study design
Case-control study.
Objectives
Investigating the association between neurodegeneration within rostral spinal cord and brain gray matter volume (GMV) and assessing the relationship between remote neurodegenerative changes and clinical outcomes at the early phase of Cervical Spondylotic Myelopathy (CSM).
Setting
University/hospital.
Methods
Using Spinal Cord Toolbox, spinal cord morphometrics (cross-sectional area [CSA], gray matter area [GMA], white matter area [WMA]) of 40 patients with CSM and 28 healthy controls (HCs) were computed and compared using two-sample t test. Brain GMV of the two groups was analyzed using voxel-based morphometry approach. Pearson’s correlation between spinal cord morphometrics and altered brain GMV and Spearman’s relationship between remote neurodegenerations and clinical outcomes were conducted in CSM group.
Results
Compared to HCs, CSA and WMA at C2/3 and GMV in right postcentral gyrus (PoCG.R) and left supplementary motor area (SMA.L) were significantly decreased in patients with CSM. CSA and WMA at C2/3 were associated with GMV in SMA.L and MCG.R in patients with CSM. CSA at C2/3 and GMV in PoCG.R were related to modified Japanese Orthopedic Association score in patients with CSM.
Conclusions
The associations between CSA and WMA at C2/3 and GMV in SMA.L and MCG.R suggest a concordant change pattern and adaptive mechanisms for neuronal plasticity underlying remote neurodegeneration in early CSM. The atrophy of CSA at C2/3 and GMV loss in PoCG.R can serve as potential neuroimaging biomarkers of early structural changes within spinal cord and brain preceding marked clinical disabilities in patients with CSM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, Yunfei Zha, on reasonable request.
Code availability
The codes that ran on the SCT and R statistical and computing software were available from the corresponding author, Yunfei Zha, on reasonable request.
References
Yarbrough CK, Murphy RK, Ray WZ, Stewart TJ. The natural history and clinical presentation of cervical spondylotic myelopathy. Adv Orthop. 2012;2012:480643.
Matz PG, Anderson PA, Holly LT, Groff MW, Heary RF, Kaiser MG, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:104–11.
Grabher P, Mohammadi S, Trachsler A, Friedl S, David G, Sutter R, et al. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci Rep. 2016;6:24636.
Seif M, Gandini Wheeler-Kingshott CA, Cohen-Adad J, Flanders AE, Freund P. Guidelines for the conduct of clinical trials in spinal cord injury: neuroimaging biomarkers. Spinal Cord. 2019;57:717–28.
Dong Y, Holly LT, Albistegui-Dubois R, Yan X, Marehbian J, Newton JM, et al. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J Neurosurg Spine. 2008;9:538–51.
Duggal N, Rabin D, Bartha R, Barry RL, Gati JS, Kowalczyk I, et al. Brain reorganization in patients with spinal cord compression evaluated using fMRI. Neurology. 2010;74:1048–54.
Grabher P, Mohammadi S, David G, Freund P. Neurodegeneration in the spinal ventral horn prior to motor impairment in cervical spondylotic myelopathy. J Neurotrauma. 2017;34:2329–34.
Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dobkin B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2007;6:544–51.
Zhou F, Gong H, Liu X, Wu L, Luk KD, Hu Y. Increased low-frequency oscillation amplitude of sensorimotor cortex associated with the severity of structural impairment in cervical myelopathy. PloS one. 2014;9:e104442.
Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013;12:873–81.
Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. Am J Roentgenol. 2011;197:W134–40.
Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Philos Pa 1976). 2001;26:1890–4.
Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100.
Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Jt Surg Am. 2013;95:1651–8.
De Leener B, Lévy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. NeuroImage. 2017;145:24–43.
Gros C, De Leener B, Badji A, Maranzano J, Eden D, Dupont SM, et al. Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. NeuroImage. 2019;184:901–15.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113.
Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51.
Huber E, David G, Thompson AJ, Weiskopf N, Mohammadi S, Freund P. Dorsal and ventral horn atrophy is associated with clinical outcome after spinal cord injury. Neurology. 2018;90:e1510–e22.
Hou JM, Yan RB, Xiang ZM, Zhang H, Liu J, Wu YT, et al. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience. 2014;266:208–15.
Liu M, Tan Y, Zhang C, He L. Cortical anatomy plasticity in cases of cervical spondylotic myelopathy associated with decompression surgery: A strobe-compliant study of structural magnetic resonance imaging. Med (Baltim). 2021;100:e24190.
Wang L, Yu B, Li Q, Qi F, Guo Q. Sensorimotor cortex atrophy in patients with cervical spondylotic myelopathy. Neuroreport. 2018;29:826–32.
Zhou Y, Shi J. Brain structural and functional dissociated patterns in degenerative cervical myelopathy: a case-controlled retrospective resting-state fMRI study. Front Neurol. 2022;13:895348.
Jütten K, Mainz V, Schubert GA, Fabian Gohmann R, Schmidt T, Ridwan H, et al. Cortical volume reductions as a sign of secondary cerebral and cerebellar impairment in patients with degenerative cervical myelopathy. Neuroimage Clin. 2021;30:102624.
Grabher P, Callaghan MF, Ashburner J, Weiskopf N, Thompson AJ, Curt A, et al. Tracking sensory system atrophy and outcome prediction in spinal cord injury. Ann Neurol. 2015;78:751–61.
Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–89.
Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–32.
Hrabálek L, Hluštík P, Hok P, Wanek T, Otruba P, Cecháková E, et al. [Effects of spinal cord decompression in patients with cervical spondylotic myelopathy oncortical brain activations]. Rozhl Chir. 2014;93:530–5.
Vogt BA. Cingulate cortex in the three limbic subsystems. Handb Clin Neurol. 2019;166:39–51.
Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–96.
Boudrias MH, Belhaj-Saïf A, Park MC, Cheney PD. Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cereb Cortex. 2006;16:632–8.
He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci. 1995;15:3284–306.
Ziegler G, Grabher P, Thompson A, Altmann D, Hupp M, Ashburner J, et al. Progressive neurodegeneration following spinal cord injury: Implications for clinical trials. Neurology. 2018;90:e1257–e66.
Rocca MA, Agosta F, Martinelli V, Falini A, Comi G, Filippi M. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. NeuroImage. 2006;30:879–84.
Chen Z, Wang Q, Liang M, Zhao R, Zhu J, Xiong W, et al. Visual cortex neural activity alteration in cervical spondylotic myelopathy patients: a resting-state fMRI study. Neuroradiology. 2018;60:921–32.
Chen Z, Zhao R, Wang Q, Yu C, Li F, Liang M, et al. Functional connectivity changes of the visual cortex in the cervical spondylotic myelopathy patients: a resting-state fMRI study. Spine (Philos Pa 1976). 2020;45:E272–e9.
Peng X, Tan Y, He L, Ou Y. Alterations of functional connectivity between thalamus and cortex before and after decompression in cervical spondylotic myelopathy patients: a resting-state functional MRI study. Neuroreport. 2020;31:365–71.
Sawada M, Nakae T, Munemitsu T, Hojo M. Functional connectivity analysis and prediction of pain relief in association with spinal decompression surgery. World Neurosurg. 2020;139:e316–e24.
Wang, C, Ellingson, BM, Islam, S, Laiwalla, A, Salamon, N, Holly, LT Supraspinal functional and structural plasticity in patients undergoing surgery for degenerative cervical myelopathy. J Neurosurg Spine. 2021;35:1-9.
Zhao R, Su Q, Chen Z, Sun H, Liang M, Xue Y. Neural correlates of cognitive dysfunctions in cervical spondylotic myelopathy patients: a resting-state fMRI study. Front Neurol. 2020;11:596795.
Zhou F, Wu L, Liu X, Gong H, Luk KD, Hu Y. Characterizing thalamocortical disturbances in cervical spondylotic myelopathy: revealed by functional connectivity under two slow frequency bands. PloS One. 2015;10:e0125913.
Zhou FQ, Tan YM, Wu L, Zhuang Y, He LC, Gong HH. Intrinsic functional plasticity of the sensory-motor network in patients with cervical spondylotic myelopathy. Sci Rep. 2015;5:9975.
Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Lange SF, et al. A novel mri biomarker of spinal cord white matter injury: T2*-weighted white matter to gray matter signal intensity ratio. Am J Neuroradiol. 2017;38:1266–73.
Acknowledgements
We would like to thank all participants for their time and effort. We are deeply grateful to programmer Wei Zhao from ShuKun Technology for her help with installing the Spinal Cord Toolbox software.
Author information
Authors and Affiliations
Contributions
CK: acquired and analyzed the data, interpreted the results, wrote the manuscript. YZ: designed the study, performed the clinical evaluation of individuals, revised the manuscript. All authors read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the ethics review board of Renmin Hospital of Wuhan University and was conducted in accordance with the Declaration of Helsinki. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuang, C., Zha, Y. Neurodegeneration within the rostral spinal cord is associated with brain gray matter volume atrophy in the early stage of cervical spondylotic myelopathy. Spinal Cord (2024). https://doi.org/10.1038/s41393-024-00971-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41393-024-00971-0