Abstract
Background
Circulating biomarkers of bone metabolism are significantly associated with overall survival (OS) in men with advanced prostate cancer. In the SWOG S1216 phase III trial, we showed that elevated bone biomarkers are significantly associated with an increased risk of death in hormone-sensitive prostate cancer (HSPC) regardless of the status of bone metastases, identifying three risk groups with differential OS outcomes based on bone biomarker status. Here we report the association of bone biomarkers with OS in men with HSPC and documented skeletal metastases as part of a planned subset analysis of S1216.
Methods
Bone resorption [C-telopeptide (CTx); Pyridinoline (PYD)] and bone formation markers [C-terminal collagen propeptide (CICP); bone alkaline phosphatase (BAP)] were assessed in blood from men with bone metastatic HSPC. Patients were randomly divided into training (n = 238) and validation (n = 475) sets. In the training set, recursive partitioning that maximizes discrimination of OS was used to identify the dichotomous cut-point for each biomarker and for a combination of biomarker split points to define prognostic groups. In the validation set, Cox proportional hazards models were used to assess the impact of biomarkers on OS, adjusted for patient and tumor characteristics.
Results
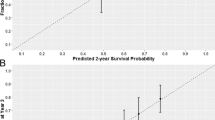
Of 1279 men, 713 had both baseline bone metastases and evaluable bone biomarkers. Patient characteristics were similar between the overall population and the subset with bone metastases. Elevated levels of CICP, CTX, and PYD were strongly prognostic for OS. Hazard ratios (95% CI) for OS adjusted for treatment arm and baseline clinical variables were: BAP–1.31 (0.93, 1.84), p = 0.12; CICP–1.58 (1.09, 2.29), p < 0.02; CTx – 1.55 (1.12, 2.15), p = 0.008; and PYD–1.66 (1.27, 2.217), p = 0.0002. There was no evidence of interaction between elevated biomarkers and treatment (all p > 0.2). Recursive partitioning algorithms identified four groups of patients with differential OS outcomes based on bone biomarkers, adjusted for baseline clinical variables, with median OS ranging from 2.3 years (highest risk group) to 7.5 years (lowest risk group).
Conclusions
In this planned S1216 subset analysis of men with HSPC and bone metastases, elevated serum markers of bone metabolism were significantly associated with worse OS. Bone biomarker levels alone and in combination with patient and tumor characteristics identify unique subsets of men with differential OS outcomes.
ClinicalTrials.gov Identifier
NCT01809691
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available by request from the SWOG Cancer Research Network and the SWOG Statistical Center. (https://www.swog.org/)
References
Whitburn J, Edwards CM. Metabolism in the tumour-bone microenvironment. Curr Osteoporos Rep. 2021;19:494–9. Oct
Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–36.
Fortunati N, Gatti F, Felicetti F, Brignardello E. Cancer treatment-induced bone loss in hormone-sensitive cancer: the paradigm of cancer survivor bone health management. Front Horm Res. 2021;54:91–102. PMID: 33946075.
Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev. 2006;32:23–6.
Lara PN Jr, Ely B, Quinn DI, Mack PC, Tangen C, Gertz E, et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst. 2014;106:dju013.
Lara PN Jr., Stadler WM, Longmate J, Quinn DI, Wexler J, Van Loan M, et al. A randomized phase II trial of the matrix metalloproteinase inhibitor BMS-275291 in hormone-refractory prostate cancer patients with bone metastases. Clin Cancer Res. 2006;12:1556–63.
Lara PN Jr, Mayerson E, Gertz E, Tangen C, Goldkorn A, van Loan M, et al. Bone biomarkers and subsequent survival in men with hormone-sensitive prostate cancer: Results from the SWOG S1216 Phase 3 trial of androgen deprivation therapy with or without Orteronel. Eur Urol. 2023;85:171–6.
Agarwal N, Tangen CM, Hussain MA, Gupta S, Plets M, Lara PN, et al. Orteronel for metastatic hormone sensitive prostate cancer: A multicenter, randomized, open-label phase III trial (SWOG-1216). J Clin Oncol. 2022;40:3301–9.
Baldessari C, Pipitone S, Molinaro E, Cerma K, Fanelli M, Nasso C, et al. Bone Metastases and Health in Prostate Cancer: From Pathophysiology to Clinical Implications. Cancers. 2023;15:1518.
Funding
Research reported herein was supported by the U.S. National Cancer Institute of the National Institutes of Health under Award Numbers CA180888 and CA180819; and in part by Millennium Pharmaceuticals (Takeda Oncology Company). PNL is supported by NCI Cancer Center Support Grant P30 CA093373 and by Jerry and Susan Knapp. This work is dedicated to the memory of Nicholas J. Vogelzang, MD.
Author information
Authors and Affiliations
Contributions
PNL conceptualized, designed, developed, and coordinated the project, led the collection and analysis of data, and served as the primary author of the manuscript. EM, CT, and MLB served as biostatisticians for this project, assisting with the methodological design, participating in the primary data analysis, and assisting in subsequent data reporting. EG and MVL performed the high-quality molecular assays and assisted in the data analysis. PNL, CT, AG, MH, DIQ, IT, and NA participated in the design and conduct of the S1216 phase III clinical trial. AG, MH, SG, JZ, MP, PT, DIQ, IT, and NA participated in accruing patients to the S1216 trial and oversaw the collection of biospecimens for this project at their respective sites. PNL, EM, and CT developed the initial manuscript draft. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented in part at the 2023 American Society of Clinical Oncology Annual Meeting in Chicago, IL.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lara, P.N., Mayerson, E., Gertz, E. et al. Markers of bone metabolism and overall survival in men with bone-metastatic hormone sensitive prostate cancer (HSPC): A subset analysis of SWOG S1216, a phase III trial of androgen deprivation with or without orteronel. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00813-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00813-3