Abstract
Background
The SPINK1 molecular subtype is more common in African-American (AA) men with prostatic adenocarcinoma (PCa) than European Americans (EA). Studies have suggested that SPINK1 expression is associated with more aggressive disease. However, the size, follow-up, and racial diversity of prior patient cohorts have limited our understanding of SPINK1 expression in AA men. The objective was to determine the associations between SPINK1 subtype, race, and oncologic outcomes after radical prostatectomy (RP).
Methods
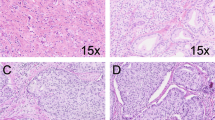
A total of 186 AA and 206 EA men who underwent RP were matched according to pathologic grade. We examined SPINK1 status by immunohistochemistry on tissue microarrays using a genetically validated assay. Cox proportional hazard analyses assessed the association of SPINK1 status with oncologic outcomes in race-specific multivariate models. A second objective was to determine the correlation between CD3/CD8 T cell densities with SPINK1 status and race, using immunostaining and automated image analysis.
Results
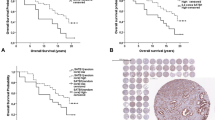
SPINK1-positive subtype was present in 25% (45/186) of AA and 15% (30/206) of EA men (p = 0.013). There were no differences in pathologic grade, pathologic stage, biochemical recurrence (BCR)-free survival, or metastasis-free survival between SPINK1-positive and SPINK1-negative tumors in the overall cohort or by race. In multivariate analyses, SPINK1 expression was not associated with BCR (AA: HR 0.99, 95% CI 0.56–1.75, p = 0.976; EA: HR 0.88, 95% CI 0.43–1.77, p = 0.720) or metastasis (AA: HR 0.79, 95% CI 0.25–2.49, p = 0.691; EA: HR 1.55, 95% CI 0.58–4.11, p = 0.381) in either AA or EA men. There were no significant differences in surrounding CD3/CD8 lymphocyte densities between SPINK1-positive and SPINK1-negative tumors in either race.
Conclusions
SPINK1-positive subtype is more prevalent in AA than EA men with PCa. Contrary to previous studies, we found that SPINK1 protein expression was not associated with worse pathologic or oncologic outcomes after RP in either AA men or EA men.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yamoah K, Johnson MH, Choeurng V, Faisal FA, Yousefi K, Haddad Z, et al. Novel biomarker signature that may predict aggressive disease in African American men with prostate cancer. J Clin Oncol. 2015;33:2789–98.
Tosoian JJ, Almutairi F, Morais CL, Glavaris S, Hicks J, Sundi D, et al. Prevalence and prognostic significant of PTEN loss in African-American and European-American men undergoing radical prostatectomy. Eur Urol. 2017;71:697–700.
Kornberg Z, Cooperberg MR, Spratt DE, Feng FY. Genomic biomarkers in prostate cancer. Trans Androl Urol. 2018;7:459–71.
Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–57.
Noone AM, Howlander N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute.
U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Visualiation Data Tool, based on November 2017 submission data (1999–2015). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute.
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67:7–30.
Magi-Galluzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, Esgueva R, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American, and Japanese patients. Prostate. 2011;71:489–97.
Khani F, Mosquera JM, Park K, Blattner M, O’Reilly C, MacDonald TY, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20:4925–34.
Huang FW, Mosquera JM, Garofalo A, Oh C, Baco M, Amin-Mansour A, et al. Exome sequencing of African-American prostate cancer reveals loss-of-function ERF mutations. Cancer Discov. 2017;7:973–83.
Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S, et al. The role of SPINK1 in ETS rearrangement negative prostate cancers. Cancer Cell. 2008;13:519–28.
Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, Andry CD, et al. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–25.
Ateeq B, Tomlins SA, Laxman B, Asangani IA, Cao Q, Cao X, et al. Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med. 2011;3:72ra17. https://doi.org/10.1126/scitranslmed.3001498.
Faisal FA, Sundi D, Tosoian JJ, Choeurng V, Alshalalfa M, Ross AE, et al. Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. Eur Urol. 2016;70:14–17.
Johnson MH, Ross AE, Alshalalfa M, Erho N, Yousefi K, Glavaris S, et al. SPINK1 defines a molecular subtype of prostate cancer in men with more rapid progression in an at risk, natural history radical prostatectomy cohort. J Urol. 2016;196:1436–44.
Tomlins SA, Alshalalfa M, Davicioni E, Erho N, Yousefi K, Zhao S, et al. Characterization of 1,577 primary prostate cancers reveals novel biological and clinicopathological insights into molecular subtypes. Eur Urol. 2015;68:555–67.
Flavin R, Pettersson A, Hendrickson WK, Fiorentino M, Finn S, Kunz L, et al. SPINK1 protein expression and prostate cancer progression. Clin Cancer Res. 2014;20:4904–11.
Grupp K, Diebel F, Sirma H, Simon R, Breitmeyer K, Steurer S, et al. SPINK1 expression is tightly linked to 6q15- and 5q21-deleted ERG-fusion negative prostate cancers but unrelated to PSA recurrence. Prostate. 2013;73:1690–8.
Huang KC, Evans A, Donnelly B, Bismar TA. SPINK1 overexpression in localized prostate cancer: a rare event inversely associated with ERG expression and exclusive of homozygous PTEN deletion. Pathol Oncol Res. 2017;23:399–407.
Terry S, Nicolaiew N, Basset V, Semprez F, Soyeux P, Maille P, et al. Clinical value of ERG, TFF3, and SPINK1 for molecular subtyping of prostate cancer. Cancer. 2015;121:1422–30.
Pan X, Zhang X, Gong J, Tan J, Yin X, Tang Q, et al. The expression profile and prognostic value of SPINK1 in initially diagnosed bone metastatic prostate cancer. Prostate. 2016;76:823–33.
Zhang X, Yin X, Shen P, Sun G, Yang Y, Liu J, et al. The association between SPINK1 and clinical outcomes in patients with prostate cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:3123–30.
Guedes LB, Antonarakis ES, Schweizer MT, Mirkeshti N, Almutairi F, Park JC, et al. MSH2 loss in primary prostate cancer. Clin Cancer Res. 2017;23:6863–74.
Kaur HB, Guedes LB, Lu J, Maldonado L, Reitz L, Barber JR, et al. Association of tumor-infiltrating T-cell density with molecule subtype, racial ancestry and clinical outcomes in prostate cancer. Mod Pathol. 2018. https://doi.org/10.1038/s41379-018-0083-x.
Smith SC, Palanisamy N, Zuhlke KA, Johnson AM, Siddiqui J, Chinnaiyan AM, et al. HOXB13 G84E-related familial prostate cancers: a clinical, histologic, and molecular survey. Am J Surg Pathol. 2014;38:615–26.
Bhalla R, Kunju LP, Tomlins SA, Christopherson K, Cortez C, Carskadon S, et al. Novel dual-color immunohistochemical methods for detecting ERG-PTEN and ERG-SPINK1 status in prostate carcinoma. Mod Pathol. 2013;26:845–48.
Lotan TL, Wei W, Morais CL, Hawley ST, Fazil L, Hurtado-Coll A, et al. PTEN loss as determined by clinical-grade immunohistochemistry assay is associated with worse recurrence-free survival in prostate cancer. Eur Urol Focus. 2016;2:180–8.
Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, et al. A prospective investigation of PTEN loss and ERG expression in lethal prostate cancer. J Natl Cancer Inst. 2016;108:djv346.
Salami SS, Hovelson DH, Kaplan JB, Mathieu R, Udager AM, Curci NE, et al. Transcriptome heterogeneity in multifocal prostate cancer. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.123468.
Wallace TA, Pruiett RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–36.
Renee RR, Agrawal D, Davis MB, Yoder S, Odedina FT, Kumar N, et al. Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: a pilot project study. Infect Agent Cancer. 2009;4(Suppl 1):S3.
Eastham JA, May RA, Whatley T, Crow A, Venable DD, Sartor O. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J Natl Cancer Inst. 1998;90:756–60.
Acknowledgements
This work was supported in part by the Department of Defense (PC141474 to EMS, SAT, and TLL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SAT has received travel support from and had a sponsored research agreement with Compendia Bioscience/Life Technologies/Thermo Fisher Scientific, which assisted in the design of the custom RNAseq panel. The University of Michigan has been issued patents on ETS gene fusions in prostate cancer, on which SAT is a coinventor. The diagnostic field of use was licensed to Hologic/Gen-Probe Inc., which has sublicensed rights to Roche/Ventana Medical Systems. SAT has served as a consultant for and received honoraria from Janssen, AbbVie, Sanofi, Almac Diagnostics, and Astellas/Medivation. SAT has sponsored research agreements with Astellas/Medivation and GenomeDX. SAT is an equity holder in and employee of Strata Oncology. TLL has received research funding from Roche/Ventana Medical Systems for other projects and has served as a consultant for and received an honorarium from Janssen. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Faisal, F.A., Kaur, H.B., Tosoian, J.J. et al. SPINK1 expression is enriched in African American prostate cancer but is not associated with altered immune infiltration or oncologic outcomes post-prostatectomy. Prostate Cancer Prostatic Dis 22, 552–559 (2019). https://doi.org/10.1038/s41391-019-0139-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0139-0
This article is cited by
-
Comparative analysis of 1152 African-American and European-American men with prostate cancer identifies distinct genomic and immunological differences
Communications Biology (2021)
-
Androgen deprivation upregulates SPINK1 expression and potentiates cellular plasticity in prostate cancer
Nature Communications (2020)