Abstract
Context
Observational studies have shown conflicting results as to whether exposure to neonatal phototherapy is associated with increased rates of childhood cancer.
Objective
To describe the rates of childhood neoplasms and cancer after neonatal phototherapy.
Data sources
The CENTRAL, PubMed, Scopus, and Web of Science databases.
Study selection
Observational studies regardless of design were included.
Data extraction
The data were extracted by one author and validated by another. The risk-of-bias assessment was performed using the ROBINS-E and Joanna Briggs Institute critical appraisal tools.
Results
Six cohort and 10 case-control studies were included. The overall risk of bias was high in seven and low in nine studies. In cohort studies, the odds ratio (OR) was increased for hematopoietic cancer (1.44; confidence interval [CI]: 1.16–1.80) and solid tumors (OR: 1.18; CI: 1.00–1.40). In case-control studies, the OR was 1.63 (CI: 0.99–2.67) for hematopoietic cancers and 1.18 (CI: 1.04–1.34) for solid tumors.
Conclusions
Children with a history of neonatal phototherapy had increased risk of hematopoietic cancer and solid tumors. The evidence quality was limited due to the high risk of bias and potential residual confounding.
Impact statement
-
Exposure to neonatal phototherapy increased later risk of hematopoietic cancer and solid tumors.
-
This is the most comprehensive study on the association between phototherapy and cancer, but the evidence quality was limited due risk of bias and residual confounding.
-
Future large scale well conducted studies are still needed to better estimate the association and.
Similar content being viewed by others
Introduction
Neonatal jaundice is a common condition during the first month of life, as approximately 70% of neonates have some level of jaundice, and 5% to 10% require phototherapy for treatment of unconjugated hyperbilirubinemia.1,2,3 Phototherapy is commonly used to decrease bilirubin levels in order to avoid the neurotoxic effects of high bilirubin levels. Some of the known risk factors for unconjugated hyperbilirubinemia requiring phototherapy are maternal red blood cell antibodies, prematurity, birth injuries, hereditary factors (ethnicity and a history of phototherapy in older siblings), and maternal obesity.3,4,5
Phototherapy has been associated with some short-term adverse events, such as rash, dehydration, and difficulties with breastfeeding,6,7 as well as with long-term risks, such as allergies and seizure disorders.8,9,10 Phototherapy has been suggested to cause DNA damage and promote reactive oxygen species and proinflammatory cytokines, which could lead to an increased cancer risk.11 In addition, phototherapy has been associated with increased incidence of café-au-lait macules in children but not with melanocytic nevi.12,13 Previous studies have shown conflicting results regarding the possible increased incidence of childhood cancers following neonatal phototherapy. In some cohort studies, children exposed to phototherapy had an increased risk of all childhood cancers,14,15 whereas no such excess was reported in other studies.16,17 It has also been speculated that there may be an association between hyperbilirubinemia and malignancies. Therefore, the association between phototherapy may be due to higher bilirubin levels or other maternal/neonatal factors that increase the risk for both hyperbilirubinemia and neoplasms. As phototherapy is an effective and frequently used therapy for neonatal unconjugated hyperbilirubinemia,18 evidence summaries on possible long-term risk are of clinical relevance. A recent meta-analysis reported an increased risk for solid cancers among children treated with phototherapy, but the authors included benign nevi in their analysis and pooled case-control and cohort studies together, which caused a notable heterogeneity in their results.19 The aim of this systematic review was to provide a systematic assessment of the incidence of cancer and neoplasms after neonatal phototherapy.
Methods
Search process
The literature search was performed on June 28, 2022. We searched the PubMed (MEDLINE), Web of Science, CENTRAL, and Scopus databases for these search terms: (neonat* OR newborn* OR infant*) AND (phototherapy OR hyperbilirubinemia OR jaundice) AND (cancer or malign* OR leukemia OR leukaemia OR lymphoma* OR tumor* or neoplasm*). Additional articles were included if found in the references of the included articles and assessed suitable for the review and analysis. We did not search other sources and decided not to include gray literature. The full search strategy is presented in the appendix (Supplementary file 1).
Inclusion criteria
We included only human studies published in peer-reviewed journals in English. Retrospective and prospective observational studies with control groups, regardless of the design (cohort, case-control, etc.) were included. Studies focusing on benign and/or malignant neoplasms, leukemia, and lymphomas were included.
Exclusion criteria
We excluded studies focusing only on nevi or other benign tumors (including hemangiomas). All animal studies were also excluded. Studies without original data or reported in languages other than English were excluded as well.
Main outcome
Our main outcome was neoplasm and cancer risk estimates stratified by anatomic site and the cell type of the neoplasm. We aimed to collect the mortality due to cancers.
Data extraction
Two authors screened the abstracts and full texts using Covidence software.20 A third party was consulted in cases of disagreement if mutual consensus was not achieved. Data extraction was performed by one author and validated by another. The following information was extracted to a pre-designed spreadsheet: authors, year of publication, country where the study was conducted, study period, study design, original inclusion criteria, exposure and control, total number of people included in the study, number of exposed and unexposed or number of cases and controls (depending on the study design), follow-up duration, and overall person-years of follow-up. The effect estimates from both adjusted and unadjusted analyses (hazard ratios [HRs], incidence rates, odds ratio [ORs], and risk ratios [RRs]) with uncertainty estimates (95% confidence intervals [CIs]) were abstracted as well.
Risk-of-bias assessment
Risk of bias was assessed for all the included studies. We used the Risk of Bias in Non-randomized Studies of Exposures (ROBINS-E) tool to assess risk of bias.21 If the study did not attempt to adjust for confounding, it was immediately labeled as high risk for bias, and other domains were not assessed. The scale used in the judgment was low, some concerns, and high. We also utilized a secondary risk-of-bias assessment strategy. We analyzed the cohort studies’ risk of bias according to the Joanna Briggs Institute critical appraisal tool for cohort studies and the case-control studies’ according to the Joanna Briggs Institute critical appraisal tool for case-control studies.22 These were labeled as with concerns or no concerns. We decided not to exclude any reports from the synthesis due to risk of bias but performed sensitivity analyses where these were excluded.
Statistical methods
RevMan version 5.4 and R statistical software version 4.2.2 (metafor package) were used for the meta-analysis. Data analysis was performed according to Cochrane Handbook for Systematic Reviews guidelines. Forest plots are presented for all outcomes.
We decided not to pool case-control studies with cohort studies, as these have different inclusion strategies and are thus problematic to combine. Overall, we expected heterogeneity in the populations between the studies, and therefore we decided to use the random-effects Mantel-Haenszel model.23 Pooled ORs with 95% CIs were calculated with the Mantel-Haenszel method for cohort and case-control studies. The inconsistency index statistic I² for statistical heterogeneity was calculated, but it was not used to decide whether the fixed-effect or random-effect model was used. Some of the studies contained outcomes that could not be pooled for quantitative analysis, and these outcomes have been reported according to the Synthesis Without Meta-analysis (SWiM) guideline.24 For example, the adjusted effect estimates in the included studies had high heterogeneity (confounder selection, statistical method, chosen effect estimate measure [OR, RR, HR]) in the reporting, and thus we decided not to force this to a single estimate and presented these in a table. We assessed publication bias by Egger’s test and the trim and fill method and provide the funnel plots.25
We report our meta-analysis according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items in Systematic Reviews and Meta-analyses (PRISMA) guidelines and provide the checklists in the appendix.26,27
Protocol registration
We registered our protocol in Prospero (ID CRD42022342273), and it can be assessed online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022342273.
Results
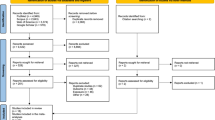
We initially screened 2,325 abstracts and assessed 31 full reports. After exclusions (19 studies) and inclusions from hand searches (4 studies), a total 16 studies were included for systematic review and meta-analysis Figure 1.14,15,16,28,29,30,31,32,33,34,35,36,37,38,39,40 Six were retrospective cohort studies and 10 case-control studies (Table 1). Eight of the studies were from Europe, five from North America, and three from the Middle East. The study periods ranged from the 1960s to the 2010s. The main outcome used was the odds or risk of any cancer. The number of participants varied between 150 and 0.9 million (Table 2). Six studies did not adjust their analysis, and, furthermore, only five studies described a rationale for the selection of the covariates for adjustments (Table 2).
Risk of bias and publication bias
Risk of bias was assessed by ROBINS-E; nine studies were judged to have a low risk of bias, and seven studies had a high risk of bias due to lack of adjustment for potential confounders (Table 3). Concerns were found in nine studies with the Joanna Briggs Institute critical appraisal tool. Most issues were in confounder identification and strategies to address incomplete follow-up in cohort studies. In case-control studies, most issues were in measuring the exposure and appropriate statistical analysis (Table 3). We did not detect publication bias visually in funnel plots, and Egger’s test confirmed this. The trim and fill method was utilized and showed no obvious asymmetry (Fig. S1).
Cancer and tumor incidence in cohort studies
Six cohort studies with a combined follow-up of 16 million person-years were analyzed and pooled for all cancer incidence estimates. In analysis by cancer type, the risk of hematopoietic cancers (OR: 1.44; CI: 1.16–1.80) and solid tumors (OR: 1.18; CI: 1.00–1.40) was increased. Rates of solid tumors and skin cancers did not show evidence of difference in crude analysis (Fig. 2). In sensitivity analyses, in which studies with high risk of bias were excluded, the OR changed only for skin cancers, and risk remained highly imprecise (OR: 1.78; CI: 0.70–7.97) (Fig. S2).
In adjusted analyses of the cohort studies, statistically significant associations were detected in two studies regarding all cancer incidences (Table 4). In stratified analysis, one study found an increased overall adjusted hazard of hematopoietic cancers and one an increased adjusted OR (aOR) for acute myeloid leukemia. One study further presented an increased aOR for kidney cancer but not for any other type of solid cancer.
Cancer and tumors in case-control studies
Ten case-control studies were included for a pooled analysis with 10,799 cancer cases, of whom 734 (7.0%) had been exposed to phototherapy. The control group consisted of 219,364 children, of whom 11,262 (5.1%) were exposed to phototherapy. In the analysis by tumor type, solid tumors were the only group with increased risk associated with phototherapy (OR: 1.18; CI: 1.04–1.34) (Fig. 3). This estimate remained unchanged in sensitivity analysis (Fig. S3). The OR for hematopoietic cancers was 1.63 (CI: 0.99–2.67). In the sensitivity analysis, the OR for hematopoietic cancers was 1.70 (CI: 1.14–2.55) (Fig. S3), indicating increased odds, when only studies with a low risk of bias were included.
Four case-control studies presented adjusted analyses. In the adjusted analyses, the aOR was statistically significant in one study and for only one outcome. The acute lymphatic leukemia aOR was 1.69 (CI: 1.37–2.08). Other adjusted estimates had CIs overlapping 1 (Table 4).
Discussion
Main findings
Based on this systematic review and meta-analysis, children with a history of neonatal phototherapy have a 1.2- to 1.6-fold increased risk of hematopoietic cancers and solid tumors. However, several factors need to be considered in interpretation, including issues with the quality of reporting in the original studies, potential causal pathways, and confounding factors.
Some studies have speculated that the increased cancer risk could be at least partly attributable to hyperbilirubinemia instead of phototherapy, i.e., confounding by indication. This could be related to oxidative stress caused by bilirubin at the cellular level, which could promote carcinogenesis.41 This is consistent with findings showing that cancer incidence among children with hyperbilirubinemia who did not receive phototherapy was between that of children without hyperbilirubinemia and that of those treated with phototherapy.15,40
We originally intended to analyze cancer risk by duration and intensity of phototherapy, as it could be hypothesized that longer treatment duration could lead to higher risk. However, it turned out that most studies did not report the phototherapy duration.
Prematurity has been associated with both phototherapy and cancer risk. One of the included studies analyzed term and preterm infants separately and found that incidence did not differ between the treated and non-treated individuals who were born prematurely, whereas among full-term infants phototherapy was associated with a slightly increased risk of hematopoietic cancers.33
Comparison to previous meta-analyses
During our initial search process, we identified a previous meta-analysis, and later another one was identified.19,42 Their results were generally similar to ours, but there were some key differences and issues in the previous meta-analyses. Both previous meta-analyses pooled case-control and cohort studies and reported their combined results. Although this is technically possible, it increases variability in study populations and adds to heterogeneity. Furthermore, the meta-analysis by Hemati et al. also included benign nevi count as an outcome and did not present any sensitivity analysis to assess the impact of risk of bias or reasons for high heterogeneity. Furthermore, we were able to include one additional study to the meta-analysis by Abdellatif et al.
Strengths
We performed our systematic review according to a pre-registered protocol without major deviations. In contrast to previous studies, we did not pool results from case-control and cohort studies, which reduces the heterogeneity in our reporting. The results from case-control studies exhibited high variability, including both increased and decreased odds. Furthermore, the measured inconsistency was high. The effect estimates from cohort studies had lower heterogeneity, which was also seen as higher statistical consistency. It must be noted that, based on the wide CIs, nearly all the included studies seemed to be underpowered to detect meaningful risk increases.
Limitations
Most of the limitations of this work come from the limitations of the included studies. Several studies had a high risk of bias due to lack of adjustment for possible confounders. The studies that did adjust for confounders rarely presented the rationale for the covariate selection. None of the studies discussed causal pathways or visualized them, e.g., as directed acyclic graphs. To overcome this issue, we have visualized the potential causal pathways in Figure S4 to better illustrate the possible causality and alternative backdoor paths causing bias to estimates.
We were unable to perform two analyses planned in the protocol: mortality and exposure-outcome gradient (dose dependency). As the studies did not report mortality, we were unable to assess it. Furthermore, we aimed to examine the exposure gradient (higher risk with higher exposure level) in the potential association, as it could have strengthened the plausibility of a potential effect. Dose dependency would have been addressed by examining the duration and intensity of the phototherapy, but only two studies presented information on duration and none on the intensity (number of lamps). Furthermore, we were unable to find information on the phototherapy practices in the included countries during the study periods, as there may have been variations in the bilirubin levels for phototherapy initiation and ending. Thus, this causes additional heterogeneity in our estimates.
Implications for clinical practice and future research
Future studies are still needed. Although our systematic review identified 16 studies, the overall quality had clear limitations. Furthermore, due to the rare outcome, estimates in our meta-analysis have notable imprecision, and further large-scale studies are needed. Future studies should focus more on potential causal pathways in selecting the covariates for their analyses. We have illustrated the potential causal pathways and modifiers, which could partly explain the observed differences (Fig. S4). Some maternal and neonatal conditions, such as prematurity, congenital anomalies, hereditary syndromes, and intrauterine growth restrictions, may increase the rates of phototherapy and cancers. Inability to control for these creates a potential source of bias due to confounding by indication and shared risk factors. Mortality in cancer patients with and without prior phototherapy would be an interesting topic to address in the future.
While our results suggest that neonatal phototherapy may increase the risk of hematopoietic cancers and solid tumors, they do not justify changes in the use of phototherapy. As high bilirubin levels are neurotoxic, it is important to treat hyperbilirubinemia appropriately. However, guidelines should be followed and unnecessary therapy avoided, as it may have harmful effects.43 Currently, we cannot conclude whether the phototherapy, high bilirubin, or shared risk factors for prematurity and childhood cancer underlie the observed association with cancer risk.
Conclusion
Neonates receiving phototherapy have a 1.2- to 1.6-fold increased risk of hematopoietic cancers and solid tumors. Quality concerns in the reporting of the original studies limited the evidence. More high-quality studies are needed to further elucidate the observed association between phototherapy and neoplasia and improve understanding of the potential causal pathways.
Data availability
All the data generated during the review process are available from the corresponding author upon request.
References
van der Geest, B. A. M. et al. Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study. Sci. Rep. 12, 14385 (2022).
Kuzniewicz, M. W., Escobar, G. J. & Newman, T. B. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 124, 1031–1039 (2009).
Mitra, S. & Rennie, J. Neonatal jaundice: aetiology, diagnosis and treatment. Br. J. Hosp. Med Lond. Engl. 2005 78, 699–704 (2017).
Kuitunen, I., Huttunen, T. T., Ponkilainen, V. T. & Kekki, M. Incidence of obese parturients and the outcomes of their pregnancies: A nationwide register study in Finland. Eur. J. Obstet. Gynecol. Reprod. Biol. 274, 62–67 (2022).
Egami, N., Muta, R., Korematsu, T. & Koga, H. Mild neonatal complications following guideline-compliant vacuum-assisted delivery in Japan: improvements still needed. J. Matern-Fetal Neonatal Med J. Eur. Assoc. Perinat. Med Fed. Asia Ocean Perinat. Soc. Int Soc. Perinat. Obstet. 35, 3400–3406 (2022).
Faulhaber, F. R. S., Procianoy, R. S. & Silveira, R. C. Side Effects of Phototherapy on Neonates. Am. J. Perinatol. 36, 252–257 (2019).
Maisels, M. J. & McDonagh, A. F. Phototherapy for neonatal jaundice. N. Engl. J. Med. 358, 920–928 (2008).
Kuniyoshi, Y., Tsujimoto, Y., Banno, M., Taito, S. & Ariie, T. Neonatal jaundice, phototherapy and childhood allergic diseases: An updated systematic review and meta-analysis. Pediatr. Allergy Immunol. Publ. Eur. Soc. Pediatr. Allergy Immunol. 32, 690–701 (2021).
Newman, T. B., Wu, Y. W., Kuzniewicz, M. W., Grimes, B. A. & McCulloch, C. E. Childhood Seizures After Phototherapy. Pediatrics 142, e20180648 (2018).
Maimburg, R. D., Olsen, J. & Sun, Y. Neonatal hyperbilirubinemia and the risk of febrile seizures and childhood epilepsy. Epilepsy Res 124, 67–72 (2016).
Oláh, J., Tóth-Molnár, E., Kemény, L. & Csoma, Z. Long-term hazards of neonatal blue-light phototherapy. Br. J. Dermatol. 169, 243–249 (2013).
Lai, Y. C. & Yew, Y. W. Neonatal Blue Light Phototherapy and Melanocytic Nevus Count in Children: A Systematic Review and Meta-Analysis of Observational Studies. Pediatr. Dermatol 33, 62–68 (2016).
Wintermeier, K. et al. Neonatal blue light phototherapy increases café-au-lait macules in preschool children. Eur. J. Pediatr. 173, 1519–1525 (2014).
Wickremasinghe A. C., Kuzniewicz M. W., Grimes B. A., Mcculloch C. E., Newman T. B. Neonatal phototherapy and infantile cancer. Pediatrics. 137. https://doi.org/10.1542/peds.2015-1353 (2016).
Auger, N., Laverdière, C., Ayoub, A., Lo, E. & Luu, T. M. Neonatal phototherapy and future risk of childhood cancer. Int J. Cancer J. Int Cancer 145, 2061–2069 (2019).
Digitale J. C., Kim M. O., Kuzniewicz M. W., Newman T. B. Update on Phototherapy and Childhood Cancer in a Northern California Cohort. Pediatrics. 148. https://doi.org/10.1542/peds.2021-051033 (2021).
Newman T. B., et al. Retrospective cohort study of phototherapy and childhood cancer in Northern California. Pediatrics .137. https://doi.org/10.1542/peds.2015-1354 (2016).
Kumar, P., Chawla, D. & Deorari, A. Light‐emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst. Rev. 2011, CD007969 (2011).
Hemati, Z., Keikha, M., Khoshhali, M. & Kelishadi, R. Phototherapy and risk of childhood cancer: A systematic review and meta-analysis. J. Neonatal Nurs. 28, 219–228 (2022).
Harrison, H., Griffin, S. J., Kuhn, I. & Usher-Smith, J. A. Software tools to support title and abstract screening for systematic reviews in healthcare: an evaluation. BMC Med Res Methodol. 20, 7 (2020).
ROBINS-E Development Group. Risk of bias tools - ROBINS-E tool. Published June 1, 2022. Accessed September 19, https://www.riskofbias.info/welcome/robins-e-tool (2022).
Zeng, X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid.-Based Med. 8, 2–10 (2015).
Greenland, S. & Robins, J. M. Estimation of a common effect parameter from sparse follow-up data. Biometrics 41, 55–68 (1985).
Campbell M., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. Published online January 16;l6890. https://doi.org/10.1136/bmj.l6890 (2020).
Lin, L. & Chu, H. Quantifying publication bias in meta-analysis. Biometrics 74, 785–794 (2018).
Page M. J., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. Published online March 29, n160. https://doi.org/10.1136/bmj.n160 (2021).
Stroup, D. F. et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 283, 2008–2012 (2000).
Auger, N., Ayoub, A., Lo, E. & Luu, T. M. Increased risk of hemangioma after exposure to neonatal phototherapy in infants with predisposing risk factors. Acta Paediatr. 108, 1447–1452 (2019).
Berg, P. & Lindelöf, B. Is phototherapy in neonates a risk factor for malignant melanoma development? Arch. Pediatr. Adolesc. Med 151, 1185–1187 (1997).
Brewster, D. H. et al. Risk of skin cancer after neonatal phototherapy: Retrospective cohort study. Arch. Dis. Child 95, 826–831 (2010).
Bugaiski-Shaked A., Shany E., Mesner O., Sergienko R., Wainstock T. Association Between Neonatal Phototherapy Exposure and Childhood Neoplasm. J Pediatr. Published online https://doi.org/10.1016/j.jpeds.2022.01.046 (2022).
Cnattingius, S. et al. Prenatal and Neonatal Risk Factors for Childhood Myeloid Leukemia. Cancer Epidemiol. Biomark. Prev. 4, 441–445 (1995).
Heck, J. E. et al. Phototherapy and childhood cancer: Shared risk factors. Int. J. Cancer J. Int. Cancer 146, 2059–2062 (2020).
Kadivar, M., Sangsari, R., Saeedi, M. & Tehrani, S. G. Association between neonatal phototherapy and cancer during childhood. Iran J. Neonatol. 11, 104–108 (2020).
Sabzevari, F., Sinaei, R., Bahmanbijari, B., Dehghan Krooki, S. & Dehghani, A. Is neonatal phototherapy associated with a greater risk of childhood cancers? BMC Pediatr. 22, 356 (2022).
Seppälä, L. K., Vettenranta, K., Leinonen, M. K., Tommiska, V. & Madanat-Harjuoja, L. Preterm birth, neonatal therapies and the risk of childhood cancer. Int J. Cancer J. Int Cancer 148, 2139–2147 (2021).
Cnattingius, S. et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J. Natl. Cancer Inst. 87, 908–914 (1995).
Linet, M. S. et al. Maternal and perinatal risk factors for childhood brain tumors (Sweden). Cancer Causes Control CCC 7, 437–448 (1996).
Roman, E., Ansell, P. & Bull, D. Leukaemia and non-Hodgkin’s lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br. J. Cancer 76, 406–415 (1997).
Podvin, D., Kuehn, C. M., Mueller, B. A. & Williams, M. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr. Perinat. Epidemiol. 20, 312–322 (2006).
Inoguchi, T., Nohara, Y., Nojiri, C. & Nakashima, N. Association of serum bilirubin levels with risk of cancer development and total death. Sci. Rep. 11, 13224 (2021).
Abdellatif, M. et al. Association between neonatal phototherapy and future cancer: an updated systematic review and meta-analysis. Eur. J. Pediatr. 182, 329–341 (2023).
Overview | Jaundice in newborn babies under 28 days | Guidance | NICE. Published May 19, 2010. Accessed March 6, https://www.nice.org.uk/guidance/cg98 (2023).
Olsen, J. H., Hertz, H., Kjaer, S. K., Bautz, A. Mellemkjaer, L. & Boice, J. D. Jr. Childhood leukemia following phototherapy for neonatal hyperbilirubinemia (Denmark). Cancer Causes Control. 7, 411–4 (1996).
Funding
This study did not receive specific funding. Open access costs will be covered by University of Eastern Finland library. Open access funding provided by University of Eastern Finland (including Kuopio University Hospital).
Author information
Authors and Affiliations
Contributions
Dr. Ilari Kuitunen had the original idea and conceptualized the study design, participated in screening process and data extraction process, was in charge of the statistical analyses, and wrote the initial draft. Dr. Atte Nikkilä participated in the conceptualization and provided methodological assistant for analyses and conducted some parts of the analyses, participated in the screening and data extraction process, and also provided important revisions to the manuscript. Dr. Johanna Jääskeläinen and Dr. Panu Kiviranta both participated in the screening process and data extraction process and provided important revisions to the manuscript. Prof. Anssi Auvinen participated in the conceptualization and supervised the whole process, provided methodological knowledge and have revised important intellectual content to the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuitunen, I., Nikkilä, A., Kiviranta, P. et al. Risk of childhood neoplasms related to neonatal phototherapy- a systematic review and meta-analysis. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03191-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03191-7