Abstract
Background
Effective seizure detection is important however, clinical signs of seizure activity may be subtle in neonates. This study aimed to systematically investigate SpO2 and respiratory pattern changes associated with EEG seizures in term-born neonates.
Method
An observational study in term neonates at risk of seizures admitted to a single tertiary level neonatal intensive care unit. Synchronised high-resolution physiological data (ECG, pulse oximetry, respiration) and EEG/amplitude-integrated EEG (aEEG) monitoring were recorded. Sections of traces with evidence of clear EEG seizure activity were compared with physiological data recorded at the same time.
Results
22/44 (50%) neonates who had aEEG monitoring were noted to have electrographic seizures. Physiologic download measurements were available for 11 of these neonates. In nine of these, an acute drop in oxygen saturation (SpO2) of at least 5% was noted in at least one seizure. Accompanying apnoeas were noted in three neonates.
Conclusion
Acute decreases in SpO2 were seen in term neonates associated with seizures and these were not always accompanied by an apnoeic episode. Physiologic download in association with EEG monitoring may assist in improving seizure detection. Unexplained drops in SpO2 could indicate further investigation for possible seizures in at-risk neonates.
Impact
-
A decrease in blood oxygen saturation (SpO2) associated with EEG seizures can occur in term infants with HIE or perinatal stroke.
-
Drops in SpO2 associated with EEG seizures in term infants with HIE or stroke may occur in the absence of apnoeas.
-
Unexplained acute falls in SpO2 in sick neonates may suggest possible seizures.
-
Drops in SpO2 associated with seizures in term infants can occur over less than 3 minutes.
-
Physiological monitoring alongside EEG monitoring could help to improve seizure detection.
Similar content being viewed by others
Introduction
Seizures commonly occur in neonates with conditions such as hypoxic-ischaemic encephalopathy and neonatal stroke.1 Prolonged uncontrolled seizures may constitute a neurologic emergency,2 and hence effective seizure detection is important. Signs of clinical seizure activity may be subtle in neonates and the relationship between clinical and electrographic seizures is poor with a large proportion of electrical seizures having no clinical manifestation.3
Continuous multichannel video-EEG is considered the gold standard for monitoring of seizures in neonates, however, the practical and interpretational challenges as well as resource implications of multichannel video-EEG limits its availability. Two-channel digital EEG monitoring alongside amplitude-integrated EEG (aEEG), is a practical and effective method for continuous neonatal EEG monitoring for the detection of seizures and assessing electrocortical background.4,5,6 Thus most centres in the UK rely on such monitors for continuous monitoring of neonates at risk of seizures,7 for example to monitor neonates receiving therapeutic hypothermia for hypoxic ischaemic encephalopathy (HIE).8
Seizures in preterm neonates may be associated with autonomic changes such as apnoeas,9 paroxysmal changes of heart rate,10 respiration and blood pressure.11 However, physiological changes such as oxygen saturation and respiratory patterns associated with seizures in term neonates have yet to be systematically studied. The aim of this study was to investigate whether electrographic seizures in term neonates are associated with physiological changes in respiratory patterns and oxygen saturation levels.
Patients and Methods
Neonates were enrolled into the prospective ADAPTS (Automated Detection And Prediction of Term newborn Seizures) observational study on the neonatal unit at the Royal London Hospital, London. Term neonates admitted to the NICU, who had aEEG monitoring, as directed by the clinicians in charge of the clinical care, were recruited to study.
Seizures
Two experienced neonatologists (DKS and ACK), who were blinded to the clinical information, identified unequivocal seizures independently with the aim of reaching a consensus when there was disagreement; a combination of clinical markers (for guidance) and the aEEG trace with confirmation from the raw EEG waveform were used to identify seizures. EEG seizure activity was defined as rhythmic spike and/wave activity lasting at least 10 seconds with evolution in seizure morphology and resolution.12,13 Impedance of the EEG electrodes was checked to be below 10kΩ.
Data Acquisition
Left and right (C3, P3 and C4, P4) channels of EEG were monitored where clinically indicated using Olympic Brainz Monitor (OBM) cerebral function monitors (Natus Medical Incorporated, Middleton, Wisconsin). The two-channel EEG recordings were synchronised with a collection of high-resolution standard physiological measurements collected as part of routine neonatal intensive care. iCollect software (GE Medical Systems Ltd., UK) on a laptop PC connected to a GE Carescape B650 monitor (GE Medical Systems Ltd., UK) was used to acquire physiological data which were being monitored for clinical management. The physiological data recorded included, ECG, heart rate (HR), oxygen saturations (SpO2), respiratory rate (RR) and invasive blood pressure where monitored for clinical management. Oxygen saturations were measured using Masimo SET (signal extraction technology) probes (Masimo Corporation, Irvine, California) and modules in the Carescape monitor.
iCollect was used to collect ECG, oximetry plethysmography, respiration and invasive blood pressure waveforms with a sampling frequency of 300 Hz. In addition, trend data were acquired for SpO2, heart rate (from oximetry and ECG), respiratory rate and systolic, diastolic and mean blood pressure; were collected at a minimum rate of once per minute.
Data analysis
Data were analysed using iCollect, Olympic Brainz Monitor Viewer, Excel (Microsoft Corporation, Redmond, Washington) and software we developed in MATLAB (The MathWorks Inc., Natick, Massachusetts); graphs were plotted using MATLAB and Minitab v19 (Minitab LLC, State College, Pennsylvania). Sections of traces with evidence of clear EEG seizure activity were compared with physiological data recorded at the same time. In this study we examined whether drops in SpO2 of at least 5% were associated with EEG seizures; the choice of 5% was from consideration of expected normal variability in SpO2. The trend graphs were compared with waveform displays to check the integrity of the data, for example that there was little or no artefact on the oximeter plethysmogram (pleth) waveform when assessing SpO2. The pulse rate from the SpO2 oximeter sensor (PR-SpO2) was checked to have no unexplained drops and to be consistent with the heart rate from ECG (HR-ECG) as an added procedure to avoid possible oximetry artefact.
Results
Between June 2021 and June 2023–51 outborn and inborn term neonates admitted to the tertiary neonatal intensive care unit at the Royal London Hospital had two channels of EEG/aEEG monitored for at least 24 hours. Forty-four neonates were recruited to the study with informed consent of whom 22 babies had EEG-confirmed seizures. There was complete agreement between the two neonatal consultants as to which infants were having unequivocal EEG seizures; furthermore, there was agreement about the presence of EEG seizures for each of the individual episodes included in the following analysis.
Seventeen of the twenty-two term-born neonates had electrographic seizure activity observed together with simultaneous physiological measurements recordings. Of these recordings, 5 had technical issues such as artefact on the oximetry pleth trace at the time of the EEG seizures; although SpO2 values were mostly still recorded when there was artefact on the pleth trace, these data were not included in case the SpO2 reading might have been affected by the artefact. In addition, we did not include analysis in one infant who had repeated seizures with little clear break between them and for whom thus any physiological changes associated with seizures would be difficult to ascertain. Thus there were eleven neonates included in the analysis who had EEG seizures with simultaneous physiological data recorded in the first ten days after birth. The minimum recording period of EEG together with physiological monitoring was 5.5 hours with most recordings more than 24 hours long. Perinatal demographic details as well as clinical parameters, diagnoses and outcomes for these eleven neonates are shown in Tables 1 and 2 respectively.
Physiologic monitoring
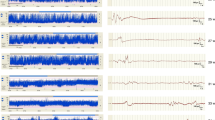
In ten of the eleven neonates with seizures, at least one EEG seizure episode was associated with a fall in SpO2 of at least 5% and for nine of whom this decrease occurred over 3 minutes or less; 3 of these babies had evidence of apnoea (≥10 s) from impedance respiration monitoring. An example of a seizure associated with a decrease in SpO2, apnoea as well as an increase in heart rate (HR) is shown in Fig. 1. This example shows an unclear increase in aEEG amplitude associated with a clear seizure on the corresponding raw EEG of 3 minutes duration which could thus potentially be missed. Figure 2 shows an example of a longer duration seizure (15 minutes) accompanied by left arm twitching and associated with a drop and some fluctuation in SpO2 over about ten minutes; in this example there was no accompanying apnoea although there is some artefact on the respiratory trace.
The upper panel a shows the left and below that right aEEG trace over a four hour period with an increase in amplitude (red vertical line) for about 3 minutes which corresponds to seizure activity on the left and right raw EEG traces (panel b). The EEG seizure activity (orange arrows) was associated with a fall in oxygen saturation (SpO2) (panel c) and a drop in respiratory rate and short apnoea (panel e) shortly after the arrow time as well as an increase in heart rate (panel d) and increase in mean arterial blood pressure (panel f); these traces are shown over a period of ten minutes. A short 30 second period of corresponding ECG, blood pressure, oximetry plethymograph (pleth) and respiration trace at the start of the apnoea is shown in panel g; there is no suggestion of artefact on either the oximetry pleth or blood pressure trace.
Panel a shows the left and below that right aEEG trace over a four hour period. There is an increase in overall amplitude of the aEEG (red vertical line) over about 15 minutes which corresponds to electrographic seizure on the raw EEG trace (panel b). The start of the EEG seizure activity, indicated with orange arrows, was associated with a fall in blood oxygen saturation (SpO2) as seen in c although there is some fluctuation in the values; a close-up of the SpO2 changes over 10 minutes is shown in d with blue dots for each individual SpO2 value. The heart rate (e) appears to increase although there is some fluctuation in earlier values and no drop in respiratory rate (f) shortly after the arrow time however, there is an increase in mean arterial blood pressure (g); traces c, e, f and g are shown over a period of fifty minutes in view of the length of the seizure activity. A short 30 second period of corresponding ECG, blood pressure, oximetry plethysmogram (pleth) and respiration trace corresponding to the seizure is shown in h in which the respiratory pattern appears variable.
Figure 3 shows an example of repeated seizures with relatively short inter-ictal periods in an infant receiving muscle paralysis for ventilation. Associated with each seizure episode was a decrease in SpO2 which was preceded by an increase in pulse rate measured by the oximeter. In the period shown after seizures there is no clear change in heart rate or SpO2.
The upper panel a shows the left and below that right aEEG trace over a four hour period with an increase in amplitude as in the example with the red vertical line which corresponds to seizure activity on the left and right raw EEG traces (panel b). The EEG seizures were associated with a fall in oxygen saturation (SpO2) (panel c) and example purple line (1) and an increase in pulse rate (panel d) which stop after the end of the seizure activity example lines 2 (orange) to 3 (green); these traces are shown over a period of sixty minutes.
Taking one measurement for each of the ten neonates who had a decrease in SpO2 associated with seizures, the median (range) fall in SpO2 was 13.5 (5 to 35)% and increase in HR was 7.5 (0 to 30) /min; six of the ten infants had invasive blood pressure recording and for mean arterial blood pressure (MABP) a median (range) increase of 6.5 (0 to 13) mmHg was seen associated with EEG seizures. One neonate had a background tachycardia (baseline HR 180 /min) and no rise in heart rate was noted associated with an EEG seizure although that infant had an SpO2 decrease of 13% and increase in MABP of 8 mmHg (Fig. 4). This infant had suffered severe multiorgan failure, was on multiple inotropes and subsequently died.
The upper panel in a shows the left and below that right aEEG trace over a four-hour period with an increase in amplitude as in the example with the red vertical line which corresponds to seizure activity on the left and right raw EEG traces plotted over 18 seconds below the aEEG. The raw ECG, blood pressure, oximetry pleth and respiratory waveforms are shown over a one-minute period in b; although the trace is compressed over 60 seconds, there is no suggestion of artefact on either the oximetry pleth trace or the blood pressure trace. The lower graphs in c, d and e show the heart rate (HR), SpO2, and mean arterial blood pressure (MABP) over 18 minutes just before and after the seizure start indicated by the green vertical arrow. In addition to the acute decrease in SpO2 (d), a preceding apnoea can be seen on the respiratory trace (b) as well as change in the pleth pattern from one with respiratory modulation to no clear respiratory modulation in the apnoeic period (b); similar changes are seen in the blood pressure trace pattern (b). There is a baseline tachycardia and no clear change in heart rate seen associated with the seizure episode (c) however, there is an increase in mean arterial blood pressure associated with the seizure (e).
Three infants with falls in SpO2 associated with seizures were receiving muscle paralysis medication as part of the ventilation support; two of these babies showed clear evidence of apnoea on the respiratory trace prior to the fall in SpO2 even though they were on synchronized intermittent mandatory ventilation (SIMV) ventilation with 100% fraction of inspired oxygen (FiO2) as seen in the example in Fig. 4. The evidence of apnoea was supported by a change in pleth signal pattern during the apnoea in which the respiratory modulation of the pleth and invasive blood pressure was absent as seen in Fig. 4; following the apnoea, respiratory modulation can again be seen on both the oximetry pleth and BP traces albeit less than before which is consistent with lower amplitude excursions on the impedance respiratory trace. The baby had a heart rate of about 180/minute and no clear change in heart rate was seen associated with the seizure episode (C); this may suggest that there was little capacity to further increase heart rate in view of the baseline tachycardia.
Nine of the ten neonates with drops in saturations were mechanically ventilated, 6 were in supplementary oxygen; all nine ventilated infants received morphine infusion for sedation.
In order to assess how often acute decreases in SpO2 can be associated with seizure activity, we reviewed the physiological and EEG recordings over a period of 5.5 to 12 hours (median 12 hours) in the ten neonates with oxygen saturation drops. The criteria for identifying an acute fall in SpO2 were that there was an SpO2 decrease of at least 5% occurring within 3 minutes and with little or no artefact on the oximetry pleth trace; the decreases were followed by a subsequent increase in SpO2 as seen in Figs. 1 to 4. Table 3 summarises the number of SpO2 falls of at least 5% together with the number associated with EEG seizures and for those the minimum and maximum drop of at least 5% in SpO2. The median (range) number of acute SpO2 falls was 7 (2 to 20) of which there were seizures in 3 (1 to 20) episodes in the review period; the median (range) percentage episodes of SpO2 falls that were associated with seizures was 50 (16 to 100) % in that period.
Discussion
We objectively demonstrate that acute drops in oxygen saturation are not uncommon in association with seizures in sick term neonates at risk of seizures and undergoing intensive care, with a drop of at least 5% oxygen saturation noted in at least one seizure in ten of eleven neonates studied; in three neonates, there were SpO2 decreases with seizures that were also associated with apnoeas as shown in Table 3. The desaturations were also noted in neonates receiving mechanical ventilation and supplementary oxygen. In addition, for the 10 babies who had falls in SpO2 associated with the EEG seizure activity, an increase in heart rate was also noted in 8 of the 10 babies and an increase in mean arterial blood pressure in 4 of 6 babies. The median (range) percentage episodes of SpO2 decreases of at least 5% that were associated with seizures was 50 (16 to 100) % in the reviewed period.
Although anecdotally reported,14 we are not aware of previous studies investigating changes in oxygen saturation and respiratory rate in term neonates with electrographic seizure activity in term infants. A recent study in preterm infants,15 also observed that some infants had falls in SpO2 associated with seizures; in that study oximetry pleth trace recording was not reported, however, we used the pleth to check for artefact that could potentially affect the indicated oxygen saturation. Apnoea, as the sole clinical manifestation associated with a seizure, has been described in neonates,16 and may be characteristic of focal epilepsies localised to the temporal lobe and associated structures.17 Ictal apnoea may be the only clinical manifestation in temporal lobe epilepsies and the apnoea may precede EEG changes and the onset of the clinical seizures in children.18 Ictal apnoeas have also been described in term neonates with neonatal temporal lobe haemorrhages.19
Plausible mechanisms of autonomic responses during seizures may be explained by changes affecting the central autonomic network (CAN), comprising of the insula and the medial prefrontal cortex, amygdala, hypothalamus and parts of the brainstem,20 which may exert control over the sympathetic and parasympathetic, neuroendocrine, respiratory and sphincter motor neurons.
Oxygen desaturations have been reported in association with seizures in children with epilepsy associated with apnoea and hypoventilation.21 In adult patients with intractable epilepsy oxygen desaturation, often in association with hypopnea and apnoea, has been noted particularly in patients with right sided temporal lobe epilepsy.22 In patients of 16 years and over, prolonged apnoeas of 60 s and greater have been associated with severe hypoxaemia with oxygen saturation <75% and have been considered at risk of sudden unexpected death in epilepsy (SUDEP).23 Furthermore using Near-Infrared Spectroscopy falls in cerebral oxygenation associated with aEEG seizures have been observed in a 35 week gestation infant.24
Interestingly, three ventilated neonates with seizures showed concomitant apparent apnoeic events on respiratory monitoring prior to falls in SpO2 including two receiving muscle paralysis for ventilation. The change in respiratory pattern detected from the impedance respiratory trace was supported by interruption in respiratory modulation of the oximetry pleth trace,25,26 together with a similar effect seen on the invasive blood pressure trace. The reason why three infants could still show marked diminution or absence of respiratory effort on monitoring despite being mechanically ventilated is not clear to us but we speculate that this could be related to ‘splinting’.27 There was no indication of a mechanical ventilation equipment problem.
There is a paucity of data in neonates who have oxygen desaturations in association with seizures. Seizure burden has been shown to be associated with brain injury in neonates who have undergone therapeutic hypothermia after HIE,8,28 and neonatal seizure burden has been shown to be associated with adverse neurologic outcomes.29,30 Although the SpO2 level at the time of the drops associated with seizures was still greater than that expected just before birth,31,32 it is unclear whether additional hypoxemic events associated with seizures could contribute to adverse outcomes in these at-risk neonates.
Limitations of this study include that using aEEG with raw EEG may miss short and focal seizures.30 However this technique has been shown to have moderately good sensitivity and specificity for seizure detection.5,33 It is less resource intensive and is applied by the nursing and medical teams relatively quickly after the admission of the baby to the neonatal unit, in circumstances where the availability of conventional EEG may be more limited; hence it is considered a pragmatic approach of enabling continuous monitoring of neonatal cerebral function which is widely adopted in the UK.7
EEG and physiological data monitored as part of standard neonatal intensive care were successfully synchronised and recorded to enable traces to be compared easily. In our approach, not only summary trends in physiological data were recorded but also the waveforms allowing increased confidence in inclusion of good quality data and exclusion of artefacts. We suggest that taking account of unexplained acute drops in SpO2 could help improve detection of seizures in vulnerable term babies particularly if the seizures are of short duration and could potentially otherwise be missed.
Conclusions
Our results indicate that term neonates with seizures not infrequently suffer from falls in oxygen saturation which may not be accompanied by an apnoeic episode; most of the infants in our study had either a diagnosis of HIE or perinatal stroke. We suggest that if unexplained decreases in oxygen saturations in at-risk term neonates are observed, seizures should be considered as possibly being part of the aetiology.
Data availability
Data are available upon reasonable request.
References
Vasudevan, C. & Levene, M. Epidemiology and aetiology of neonatal seizures. Semin. Fetal Neonatal Med. 18, 185–191 (2013).
Pavel, A. M. et al. Neonatal Seizure Management: Is the Timing of Treatment Critical? J. Pediatr. 243, 61–68.e2 (2022).
Murray, D. M. et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch. Dis. Child Fetal Neonatal Ed. 93, F187–F191 (2008).
Shah, D. K. et al. Use of 2-channel bedside electroencephalogram monitoring in term-born encephalopathic infants related to cerebral injury defined by magnetic resonance imaging. Pediatrics 118, 47–55 (2006).
Rakshasbhuvankar, A., Paul, S., Nagarajan, L., Ghosh, S. & Rao, S. Amplitude-integrated EEG for detection of neonatal seizures: a systematic review. Seizure 33, 90–98 (2015).
Hellström-Westas, L. Amplitude-integrated electroencephalography for seizure detection in newborn infants. Semin. Fetal Neonatal Med. 23, 175–182 (2018).
Mescall, S., Dhamodaran, M., Clarke, P. & Ponnusamy, V. Current availability of cerebral function monitoring and therapeutic hypothermia equipment in UK neonatal units and networks. Arch. Dis. Child Fetal Neonatal Ed. 106, 225–227 (2021).
Shah, D. K. et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed. 99, F219–F224 (2014).
Fenichel, G. M., Olson, B. J. & Fitzpatrick, J. E. Heart rate changes in convulsive and nonconvulsive neonatal apnea. Ann. Neurol. 7, 577–582 (1980).
Shah, D. K., Zempel, J., Barton, T., Lukas, K. & Inder, T. E. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr. Res 67, 102–106 (2010).
Perlman, J. M. & Volpe, J. J. Seizures in the preterm infant: effects on cerebral blood flow velocity, intracranial pressure, and arterial blood pressure. J. Pediatr. 102, 288–293 (1983).
Clancy, R. R. & Legido, A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia 28, 537–541 (1987).
Tsuchida, T. N. et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J. Clin. Neurophysiol. 30, 161–173 (2013).
Helmers, S. L., Weiss, M. J. & Holmes, G. L. Apneic seizures with bradycardia in a newborn. J. Epilepsy 4, 173–180 (1991).
Lee, S. S., El Ters, N., Vesoulis, Z. A., Zempel, J. M. & Mathur, A. M. Variable association of physiologic changes with electrographic seizure-like events in infants born preterm. J. Pediatr. 257, 113348 (2023).
Falsaperla, R., Consentino, M. C., Vitaliti, G., Marino, S. & Ruggieri, M. Isolated ictal apnea in neonatal age: Clinical features and treatment options. A systematic review. Auton. Neurosci. 243, 103034 (2022).
Lacuey, N., Hampson, J. P., Harper, R. M., Miller, J. P. & Lhatoo, S. Limbic and paralimbic structures driving ictal central apnea. Neurology 92, e655–e669 (2019).
Tio, E., Culler, G. W., Bachman, E. M. & Schuele, S. Ictal central apneas in temporal lobe epilepsies. Epilepsy Behav. 112, 107434 (2020).
Hoogstraate, S. R., Lequin, M. H., Huysman, M. A., Ahmed, S. & Govaert, P. P. Apnoea in relation to neonatal temporal lobe haemorrhage. Eur. J. Paediatr. Neurol. 13, 356–361 (2009).
Baumgartner, C., Lurger, S. & Leutmezer, F. Autonomic symptoms during epileptic seizures. Epileptic Disord. 3, 103–116 (2001).
Hewertson, J., Boyd, S. G., Samuels, M. P., Neville, B. G. & Southall, D. P. Hypoxaemia and cardiorespiratory changes during epileptic seizures in young children. Dev. Med Child Neurol. 38, 511–522 (1996).
Bateman, L. M., Li, C. S. & Seyal, M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 131, 3239–3245 (2008).
Lacuey, N. et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 59, 573–582 (2018).
Martini, S., Paoletti, V., Faldella, G. & Corvaglia, L. Cerebral oxygenation patterns during electroclinical neonatal seizures. Neuropediatrics 50, 408–409 (2019).
Wertheim, D., Parsley, C., Burgess, S., Dakin, C. & Seddon, P. Pulse oximetry plethysmogram analysis could help identify infants with possible apnoeas requiring full investigation. Acta Paediatr. 103, e222–e224 (2014).
Wertheim, D., Anton, O., Olden, C., Le Maistre, S. L. V. & Seddon, P. C. Pulse oximetry respiratory monitoring for assessment of acute childhood wheeze. Arch. Dis. Child 107, 1083–1087 (2022).
Vas, C., Medd, N. & Bustani, P. The desaturating intubated neonate: is DOPE enough? Infant 11, 47–50, www.infantjournal.co.uk/journal_article.html?id=6822 (2015).
Kharoshankaya, L. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev. Med Child Neurol. 58, 1242–1248 (2016).
Vesoulis, Z. A. et al. Early electrographic seizures, brain injury, and neurodevelopmental risk in the very preterm infant. Pediatr. Res 75, 564–569 (2014).
Basti, C. et al. Seizure burden and neurodevelopmental outcome in newborns with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: A single center observational study. Seizure 83, 154–159 (2020).
Toth, B., Becker, A. & Seelbach-Göbel, B. Oxygen saturation in healthy newborn infants immediately after birth measured by pulse oximetry. Arch. Gynecol. Obstet. 266, 105–107 (2002).
Lara-Cantón, I. et al. Oxygen saturation and heart rate in healthy term and late preterm infants with delayed cord clamping. Pediatr. Res. https://doi.org/10.1038/s41390-021-01805-y (2022).
Shah, D. K. et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics 121, 1146–1154 (2008).
Acknowledgements
We are grateful for the assistance of staff on the Neonatal Intensive Care Unit at the Royal London Hospital and to Barts Charity for support received. Our thanks also to Anurag Anurag, Neonatal Intensive Care Unit at the Royal London Hospital for technical assistance. Judy Moore (Natus Medical) and Mike Powderly (Belmont Medical Technologies Ltd.) kindly provided valuable assistance regarding the Natus OBM monitors. We are also grateful to staff at GE Healthcare for help with the iCollect software.
Funding
We are grateful for support received from Barts Charity, reference number MRC0282.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. AK, DS, ILM and CF obtained informed parental consent and acquired the data recordings. AK and DS reviewed the aEEG and EEG recordings. AK, DS and DW analysed the recorded data and wrote the initial draft manuscript. All authors read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics review
Ethics approval was granted (REC reference 20/PR/0969) for retrospective use, subject to parental consent, of the EEG and physiological data recordings.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wertheim, D., Kage, A.C., Lancoma-Malcolm, I. et al. Falls in oxygen saturations accompany electrographic seizures in term neonates: an observational study. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03063-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03063-0