Abstract
Background
Perfluoroalkyl substances (PFASs) are transferred through human milk and may cause elevated exposure during infancy. Given the lack of early postnatal blood samples, PFAS concentrations can be estimated to serve as predictors of subsequent metabolic toxicity.
Methods
A total of 298 children from a prospective birth cohort were followed up through to age 9 years. Serum-PFAS was measured at birth and 18 months of age, while exposures during infancy were estimated by structural equations. Adiponectin, resistin, leptin, and the leptin receptor were measured in serum at age 9. Adjusted regression coefficients for estimated serum-PFAS concentrations were calculated, with additional consideration of the duration of breastfeeding and potential effect modification by sex.
Results
A doubling in estimated serum-PFAS concentrations, particularly at ages 6 and 12 months, was associated with a loss of about 10–15% in age 9 resistin concentrations, while other associations were much weaker. Sex dependence of the associations was not observed, and neither did the duration of breastfeeding affect outcomes at age 9.
Conclusion
Lowered serum-resistin concentrations at age 9 years were most strongly associated with early postnatal PFAS exposures. These findings suggest that infancy may represent a vulnerable time window for some aspects of metabolic programming that may be affected by PFAS exposure.
Impact
-
Serum-PFAS concentrations during infancy can be estimated in the absence of blood samples.
-
Adipokine concentrations were measured at age 9 years as metabolic biomarkers.
-
Resistin was significantly lower in children with elevated PFAS exposures in infancy.
-
The findings suggest that early postnatal PFAS exposures may affect subsequent metabolic health.
-
Assessment of infancy vulnerability to PFAS can be explored using estimated serum-PFAS concentrations.
Similar content being viewed by others
Introduction
Prenatal exposure to a variety of adverse factors, such as maternal smoking, alcohol consumption, maternal dietary deficiency or nutrient oversupply, may affect the later health of the offspring,1 and this “fetal programming” may also be affected by toxicity due to environmental chemicals.2 Less emphasis has been placed on early postnatal exposures, although the existence of infancy vulnerability is beyond doubt.3 Thus, understanding of the temporal profile of toxicant exposures with regard to vulnerable developmental stages is crucial for public health purposes.4
Early postnatal exposure can be of particular relevance for substances that are excreted into human milk, such as perfluoroalkyl substances (PFASs).5,6 Serum-PFAS concentrations in infants breastfed for 6 or more months may increase by as much as 10-fold at the end of infancy as compared to those not breastfed.5 Among adverse health outcomes, immune functions appear to be particularly sensitive to early postnatal exposures.7 In addition, obesity seems to be initiated in childhood,8,9,10 although the age-dependent vulnerability to obesogenic or other metabolic effects is unclear. Research in this field is hampered by the frequent lack of blood samples at early ages that could be analyzed for exposure biomarkers. Also, common clinical variables, such as growth trajectories, may not be sufficiently sensitive to reveal early disruptions. Thus, certain metabolic biomarkers in serum, such as adipokines, may be useful as indicators of metabolic homeostasis, inflammation, and the possible risk of developing early signs of metabolic syndrome.11,12 These hormonal factors include leptin and its receptor, adiponectin, and resistin, which are associated with childhood growth and with the odds of being or becoming overweight and developing chronic disease.12,13,14 Serum-adipokine concentrations may be affected by other factors, e.g., preterm birth that appears to be associated with lower adiponectin and higher leptin and resistin in later childhood.14,15 In addition, the duration of breastfeeding may be of potential importance, as the presence of adipokines has been detected in human milk.16,17
Recent studies have begun to explore the possible impact of developmental PFAS exposure and serum concentrations of adipokines measured in later childhood,18 with most attention being paid to prenatal PFAS exposure and its associations with leptin and adiponectin.14,18,19,20,21,22 We previously observed longitudinal associations between PFAS exposures measured in maternal pregnancy serum or in child serum from age 5 years and serum-adipokine concentrations in childhood.23 In agreement with other recent evidence,14 we found that adipokine concentrations in cord blood were not associated with prenatal PFAS exposures. On the other hand, serum-adipokine concentrations measured at age 9 years were primarily associated with PFAS concentrations in cord blood and serum from age 18 months and only to a lesser degree with serum-PFAS at 5 and 9 years.24 These findings suggest that adipokine concentrations in later childhood may be affected by early-life exposures. As PFAS exposures after birth can increase substantially due to breastfeeding,5,25 the aim of the present study was to ascertain the possible association of childhood adipokine concentrations with serum-PFAS concentrations in infancy. Because blood samples were not available from early postnatal age, the PFAS concentrations were modeled by a structural equations approach that has been previously validated.7
Methods
Study population
The study population is a subset of a birth cohort of 490 mother–child pairs recruited between October 2007 and April 2009 from the National Hospital in Tórshavn, Faroe Islands (Cohort 5). Only singleton, full-term births with complete information were included in the present study. Blood from the cord was collected at parturition, and additional blood samples from the child were obtained at follow-up clinical examinations at ages 18 months, and 5 and 9 years, where questionnaires and physical examinations were also administered.7,26 The study protocol was approved by the Faroese ethical review committee and the Harvard T.H. Chan School of Public Health institutional review board. Written informed consent was obtained from all participating mothers.
PFAS analysis of serum samples
Exposures to five major PFASs (i.e., perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA)) were assessed from all serum samples using online solid-phase extraction followed by high-pressure liquid chromatography with tandem mass spectrometry,27 with extraction conducted using a Thermo Scientific EQuan MAX system (Thermo Scientific, San Jose, CA). High precision was suggested by within-batch and between-batch coefficients of variation at <3% and 5–6%, respectively. For participants with values below the limit of detection (0.03 ng/mL for all PFASs), a value of 0.015 ng/mL was assigned.24 The quality was confirmed by regular participation with excellent results in the German-External Quality Assessment Scheme organized by the German Society of Occupational Medicine.
Adipokine hormones
As previously described,24 adipokine hormones were measured in serum using commercial ELISA kits according to the instructions from the manufacturer. The following kits from Biovendor (Brno, Czech Republic) were used: leptin (VEN/RD191001100), soluble leptin receptor (OB-R, VEN/RD194002100), total adiponectin (VEN/RD191023100), and resistin (VEN/RD191016100). For adiponectin, all samples were diluted 1:1200 to obtain measurable levels. We evaluated the performance of the kits using a pooled donor serum obtained from the Department of Clinical Immunology at Odense University Hospital. High-performance accuracy was suggested by the low coefficients of variation of 2.9% for leptin, 2.1% for leptin receptor, 5.3% for adiponectin, and 7.4% for resistin. The results allowed calculation of the free leptin index as the ratio between leptin and leptin receptor concentrations28 as well as the adiponectin/leptin ratio.13,29
Covariables
Standard questionnaires were used in the obstetric ward to collect maternal age (years), maternal smoking during pregnancy (none, 1–5 cigarettes per day, >5 cigarettes per day), maternal education (low, medium, high), and child sex. Additional obstetric information was abstracted from hospital charts, including gestational age (weeks), parity (primiparous, multiparous) and pre-pregnancy weight, height, and body mass index (BMI, kg/m2). Duration of breastfeeding (months; i.e., exclusive, mixed, and none) was obtained from the 18-month maternal questionnaires.
Potential confounders were selected using directed acyclic graphs based on prior literature on early postnatal PFAS concentrations and childhood metabolic parameters.8,23,30 Maternal age, maternal pre-pregnancy BMI, maternal smoking during pregnancy, parity, maternal education, and child sex were considered mandatory for adjustment of all analyses.
Statistical analyses
PFAS concentrations were log-transformed, and adipokine concentrations were similarly transformed due to skewed distributions. We modeled early postnatal serum-PFAS concentrations at 3, 6, and 12 months based on breastfeeding information as well as the measured PFAS concentrations in cord serum and in the child’s serum at age 18 months.7 This was achieved by using structural equation models in an approach as previously described.7 In short, the model assumes that the log-transformed PFAS concentration changes by a slope of α during the period of exclusive breastfeeding and likewise, during partial breastfeeding, the slope is β, and after weaning, the slope is γ:
where log PFASi,a is the log-transformed serum concentration of child i at age a, while exclusivei,a, partiali,a, and nomilki,a indicate the number of months that the child was exclusively breastfed, partially breastfed, and not breastfed at all by age a. The variable Ui accounts for within-child correlation, i.e., the fact that a child above the mean at one timepoint will also tend to be higher at other time points. The last term (εi,a) is a random measurement error. According to the model, the true PFAS concentration of child i at age a is given by the latent variable Ei,a =µ + α exclusivei,a + β partiali,a + γ nomilki,a + Ui.. In the second part of the model, this variable was then considered a predictor of the serum-adipokine concentration at age 9 years by linear regression adjusted for covariates. The regression coefficients were expressed as the change for each doubling of the serum-PFAS concentrations at ages 3, 6, and 12 months. Interactions by child sex were assessed by including an interaction term between PFAS and sex in the model. We tested for this interaction (psex) based on the corresponding value of the likelihood ratio test statistic. Although the duration of exclusive and mixed breastfeeding was not associated with adipokine hormone concentrations at age 9 years (p > 0.1), we included a sensitivity analysis taking into account the duration of breastfeeding.
All regression analyses were conducted using R software, and the structural equation models were fitted using R software’s Lava package.31
Results
The present study included 298 mother–child pairs with complete data on serum-PFAS concentrations at birth and 18 months, adipokine hormones at 9 years, and important covariables; little difference was apparent when compared to the full cohort (Table 1). Most mothers were non-smokers (83%), and approximately equal numbers of boys (52%) and girls (48%) were enrolled. The characteristics differed only slightly from those of the total Cohort 5. The distributions of the serum-PFAS concentrations at birth and 18 months are shown in Table 2. All five PFAS concentrations at 18 months were substantially higher than those at birth, as previously reported,24 although 33 infants showed PFHxS at 18 months below the level of detection (and were assumed to be 0.015 ng/mL). The estimated serum-PFAS concentrations during infancy show that a substantial increase occurred during the first postnatal year (Table 2).
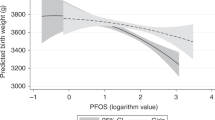
Associations of the estimated serum-PFAS concentrations at 3, 6, and 12 months with serum-adipokine concentrations at 9 years are shown in Table 3. Figure 1 compares these results with the previously obtained regression coefficients for the serum-PFAS concentrations measured at birth and at 18 months.24 For leptin, no significant associations with PFAS exposures were seen (Table 3), as was also the case with the leptin receptor and the free leptin index (Supplementary Table 1). For adiponectin, positive associations were observed for all PFASs, with a statistically significant increase for infancy-age PFHxS (Table 3). Thus, a doubling of PFHxS at age 6 months was associated with an increase of 5.0% in adiponectin at 9 years. Furthermore, the free leptin index (Supplementary Table 2) showed only marginal associations with the estimated PFAS exposures. Likewise, the adiponectin/leptin ratio15 was not associated with the exposure parameters. By far, the strongest associations were observed for resistin, where all PFASs showed significant associations during infancy (Table 3), while no clear association was observed with the measured PFOS and PFOA concentrations at age 18 months (Fig. 1). A doubling in late infancy PFAS exposure was associated with resistin decreases of about 10% or more at age 9 years.
For comparison, previously reported results24 from serum-PFAS at birth and 18 months are also shown.
Overall, these tendencies appeared substantially stronger for estimated PFAS exposures during the first year of life than those previously reported for serum-PFAS measured at birth or at later childhood ages.14, 24 Thus, although the confidence intervals were wider for the estimated PFAS values (Fig. 1), clear associations were observed for resistin in regard to infancy exposures, while only PFHxS, PFNA and PFDA showed a significant association at 18 months of age, and none at birth or at ages 5 and 9 years. Regarding interaction with sex, no statistical significance was found at infancy ages (Table 3 and Supplementary Fig. 1). In sensitivity analyses that additionally included breastfeeding in the model, only marginal changes were observed (p > 0.1).
Discussion
The present study relied on estimated serum-PFAS concentrations in infancy using a structural equation model using procedures previously described and validated.7 As a likely result of continued breastfeeding, PFAS concentrations increased during infancy. Likewise, the serum-PFAS at age 18 months showed higher concentrations than those observed prenatally or at later childhood ages 5 and 9 years,24 as shown in Table 2. The elevated exposures during early life were associated with changes in serum-adipokine concentrations at age 9 years, most strongly with reductions in resistin. No clear sex-specific association was observed between early-life exposures to PFAS in relation to the subsequent adipokine outcomes (Table 3 and Supplementary Fig. 1). Our previous study of a subset from the older Faroese Cohort 3—at much higher exposures to PFOS and PFHxS—suggested inverse associations, some being sex-dependent, between PFAS exposures and adiponectin, leptin, and resistin,23 but only the latter association was replicated at the lower serum-PFAS concentrations in the new cohort.
Due to the central role of the adipokines, the associations identified are of substantial interest regarding PFAS-associated programming of metabolic disease.32 Thus, adiponectin is reduced and leptin is upregulated in the presence of obesity,11,33 and resistin tends to be elevated in metabolic disease.34 Thus, an imbalance in the expression of the pro- and anti-inflammatory adipokines seems to affect metabolic homeostasis.12 Still, considering the possible interactions between adipokines, the PFAS-associated changes in serum concentrations at age 9 are so far difficult to interpret in detail, given the few relevant studies from infancy and childhood. However, the additional observation of decreased leptin in cord blood at elevated PFAS exposures,22,30 supports the hypothesis of early-life PFAS exposures influencing these metabolic markers in later childhood. Furthermore, studies in adults indicate that adiponectin concentrations may be affected by PFAS exposures.35,36,37 Thus, the strong associations between estimated infancy exposures to PFAS and the later adipokine concentrations, especially of resistin, support the notion that exposures occurring during early postnatal life may affect important metabolic programming processes.
The presence of adipokines in human milk may suggest that breastfeeding can also affect early metabolic programming,17 and for this reason, sensitivity analyses included adjustment for the duration of breastfeeding. No impact was identified, perhaps because adipokine concentrations in milk appear to vary and depend on a multitude of factors. Thus, although the transfer of adipokines via milk has been reported, the duration of breastfeeding seems not to be an important predictor of the child’s serum-adipokine concentrations later on. This issue is of importance, as lactation is a major elimination pathway for maternal PFAS burdens, and duration of exclusive breastfeeding is a key predictor of the child’s early postnatal exposure, particularly for PFOS and PFOA.5,25 Thus, the differential patterns of PFAS associations with the adipokines presented in this study likely reflect the changing PFAS exposure profiles early postnatally. Although this is the first study to address PFAS exposures in infancy and their associations with adipokines measured in later childhood, it had to rely on estimated serum-PFAS concentrations, as infancy serum samples were not available. The use of structural equations for this purpose has been previously used to link infancy-age PFAS exposures to deficient antibody responses to childhood vaccines later on.7 However, given that the infancy exposures were estimated and not measured, it was not possible to model the total impact of the PFASs and its temporal variation.
As a main finding in this study, the estimated infancy-age PFAS exposures tended to show stronger associations with the adipokine concentrations at age 9 than did the prenatal exposures, as observed most clearly for resistin. This adipokine seems to modulate insulin resistance and is linked to both metabolic and cardiovascular disease.34 Still, the PFAS-associated lowering of resistin at age 9 years needs further documentation to allow a more detailed interpretation. The wide confidence intervals for the estimated exposures (Fig. 1) may hide additional associations, and actual PFAS analyses of infant blood samples in the future may well result in better precision. Study protocols for new birth cohorts should therefore consider the possible inclusion of blood sampling at early postnatal ages to obtain more accurate information that may lead to better insight into early-life PFAS exposures and their possible associations with adipokine concentrations and other metabolic markers in later childhood.
A major strength of this study is the prospective study design, which allowed us to examine developmental PFAS exposures in regard to adipokine concentrations in later childhood. Such associations between variables separated in time likely minimize the possibility of reverse causation. Although some adipokine variables failed to show significant associations with the exposure data, the uniformly negative tendencies for resistin strongly support the notion that early postnatal PFAS exposure can impact later childhood adipokine status.
Early-life exposure to PFAS is considered of prime toxicological importance,2,3 and the recent guidelines for limiting PFAS exposures in the EU were developed to protect the fetus and the infant as the most vulnerable population, primarily for immunotoxicity.38 Given that human resistin is involved, e.g., in the regulation of inflammation,39 changes in resistin and perhaps other adipokines may have implications for metabolic patterns as well as immune functions that both deserve attention concerning possible early postnatal (re)programming affected by PFAS exposure.
Conclusions
In 298 children, clear, negative associations were found for their serum-resistin concentrations at age 9 years in regard to estimated infancy-age PFAS exposures; in addition, weak, positive associations were observed for the age 9 adiponectin concentration. These associations tended to be stronger than those observed with prenatal PFAS exposures; sex dependence of the associations was not observed. These findings suggest that infancy may represent a vulnerable time window for certain forms of PFAS-associated metabolic programming.
Disclaimer
The authors are solely responsible for all results and conclusions, which do not necessarily reflect the official position of the National Institute of Environmental Health Sciences of the NIH or any other funding agency. The use of trade names is for identification only and does not imply any endorsement.
Data availability
The dataset analyzed in this study is not publicly available due to national data security legislation on sensitive personal data.
References
Barker, D. J. The origins of the developmental origins theory. J. Intern. Med. 261, 412–417 (2007).
Grandjean, P. et al. Timescales of developmental toxicity impacting on research and needs for intervention. Basic Clin. Pharm. Toxicol. 125, 70–80 (2019).
Narciso, L. et al. The Juvenile Toxicity Study as a tool for a science-based risk assessment in the children population group. Reprod. Toxicol. 72, 136–141 (2017).
Heindel, J. J. The developmental basis of disease: update on environmental exposures and animal models. Basic Clin. Pharm. Toxicol. 125, 5–13 (2019).
Mogensen, U. B., Grandjean, P., Nielsen, F., Weihe, P. & Budtz-Jorgensen, E. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ. Sci. Technol. 49, 10466–10473 (2015).
van Beijsterveldt, I. et al. Poly- and perfluoroalkyl substances (PFAS) exposure through infant feeding in early life. Environ. Int. 164, 107274 (2022).
Grandjean, P. et al. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 14, 188–195 (2017).
Karlsen, M. et al. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod. Toxicol. 68, 145–153 (2017).
Jensen, R. C. et al. Prenatal exposures to perfluoroalkyl acids and associations with markers of adiposity and plasma lipids in infancy: an Odense Child Cohort Study. Environ. Health Perspect. 128, 77001 (2020).
Bloom, M. S. et al. Association between gestational PFAS exposure and children’s adiposity in a diverse population. Environ. Res. 203, 111820 (2022).
Jung, U. J. & Choi, M. S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 6184–6223 (2014).
Taouis, M. & Benomar, Y. Is resistin the master link between inflammation and inflammation-related chronic diseases? Mol. Cell Endocrinol. 533, 111341 (2021).
Yeung, E. H., Sundaram, R., Xie, Y. & Lawrence, D. A. Newborn adipokines and early childhood growth. Pediatr. Obes. 13, 505–513 (2018).
Buck, C. O. et al. Prenatal exposure to perfluoroalkyl substances and adipocytokines: The Home Study. Pediatr. Res. 84, 854–860 (2018).
Ordonez-Diaz, M. D. et al. Plasma adipokines profile in prepubertal children with a history of prematurity or extrauterine growth restriction. Nutrients 12, 1201 (2020).
Kratzsch, J., Bae, Y. J. & Kiess, W. Adipokines in human breast milk. Best. Pract. Res Clin. Endocrinol. Metab. 32, 27–38 (2018).
Badillo-Suarez, P. A., Rodriguez-Cruz, M. & Nieves-Morales, X. Impact of metabolic hormones secreted in human breast milk on nutritional programming in childhood obesity. J. Mammary Gland Biol. Neoplasia 22, 171–191 (2017).
Fleisch, A. F. et al. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ. Health Perspect. 125, 481–487 (2017).
Halldorsson, T. I. et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ. Health Perspect. 120, 668–673 (2012).
Minatoya, M. et al. Association of prenatal exposure to perfluoroalkyl substances with cord blood adipokines and birth size: The Hokkaido Study on environment and children’s health. Environ. Res. 156, 175–182 (2017).
Rifas-Shiman, S. L. et al. First and second trimester gestational weight gains are most strongly associated with cord blood levels of hormones at delivery important for glycemic control and somatic growth. Metabolism 69, 112–119 (2017).
Ding, J. et al. Associations of perfluoroalkyl substances with adipocytokines in umbilical cord serum: a mixtures approach. Environ. Res. 216, 114654 (2022).
Shelly, C. et al. Early life exposures to perfluoroalkyl substances in relation to adipokine hormone levels at birth and during childhood. J. Clin. Endocrinol. Metab. 104, 5338–5348 (2019).
Shih, Y. H., Blomberg, A. J., Jorgensen, L. H., Weihe, P. & Grandjean, P. Early-life exposure to perfluoroalkyl substances in relation to serum adipokines in a longitudinal birth cohort. Environ. Res. 204, 111905 (2022).
Verner, M. A. et al. A simple pharmacokinetic model of prenatal and postnatal exposure to perfluoroalkyl substances (PFASS). Environ. Sci. Technol. 50, 978–986 (2016).
Weihe, P. et al. Overview of ongoing cohort and dietary studies in the Arctic. Int. J. Circumpolar Health 75, 33803 (2016).
Eryasa, B. et al. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ. Int. 130, 104874 (2019).
Kratzsch, J. et al. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J. Clin. Endocrinol. Metab. 87, 4587–4594 (2002).
Li, N. et al. Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ. Int. 147, 106344 (2021).
Domazet, S. L. et al. Exposure to perfluoroalkylated substances (PFAS) in relation to fitness, physical activity, and adipokine levels in childhood: the European Youth Heart Study. Environ. Res. 191, 110110 (2020).
Holst, K. K. & Budtz-Jørgensen, E. Linear latent variable models: The Lava-Package. Comput. Stat. 28, 1385–1452 (2013).
Braun, J. M. Early-life exposure to EDCS: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 13, 161–173 (2017).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 (2011).
Park, H. K., Kwak, M. K., Kim, H. J. & Ahima, R. S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 32, 239–247 (2017).
Liu, G. et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLoS Med. 15, e1002502 (2018).
Cardenas, A. et al. Association of perfluoroalkyl and polyfluoroalkyl substances with adiposity. JAMA Netw. Open 1, e181493 (2018).
Bassler, J. et al. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ. Pollut. 247, 1055–1063 (2019).
European Food Safety Authority. Vol. EFSA Journal 18(9) (ed. Panel on Contaminants in the Food Chain) (EFSA,, 2020).
Tripathi, D., Kant, S., Pandey, S. & Ehtesham, N. Z. Resistin in metabolism, inflammation, and disease. FEBS J. 287, 3141–3149 (2020).
Acknowledgements
We are extremely grateful to all the families who took part in this study and the staff and colleagues who helped during the different phases of the cohort study.
Funding
This work was supported by the National Institute of Environmental Health Sciences (ES026596 and ES027706); the Danish Environmental Protection Agency as part of the environmental program Danish Cooperation for Environment in the Arctic (DANCEA); and the Faroese Research Council. Open access funding provided by University Library of Southern Denmark.
Author information
Authors and Affiliations
Contributions
P.G., Y.-H.S. and E.B.-J. conceived the idea for this manuscript. P.W. supervised the clinical examinations. F.N. determined the PFAS concentrations and L.H.J. measured the adipokines in the blood samples. Y.-H.S., E.B.-J. and P.G. conducted the statistical analysis. P.G. wrote the first draft, and all authors contributed to the final version.
Corresponding author
Ethics declarations
Competing interests
P.G. has provided paid expert assistance in legal cases involving PFAS-exposed populations. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
All participants provided assent at the age 9 visit, and a parent provided informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grandjean, P., Shih, YH., Jørgensen, L.H. et al. Estimated exposure to perfluoroalkyl substances during infancy and serum-adipokine concentrations in later childhood. Pediatr Res 94, 1832–1837 (2023). https://doi.org/10.1038/s41390-023-02665-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02665-4