Abstract
Coronavirus disease 2019 in children presents with milder clinical manifestations than in adults. On the other hand, the presence of a wide range of inflammatory manifestations, including multisystem inflammatory syndrome in children (MIS-C), in the period after infection suggests a particular susceptibility of some children toward severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Both protective factors that prevent evolution to severe forms and risk factors for post-infectious conditions are likely to be found in age-related differences in the immune system. The prompt innate response with type I IFN production and the generation of neutralizing antibodies play a crucial role in containing the infection. The greater number of naive and regulatory cells in children helps to avoid the cytokine storm while the causes of the intense inflammatory response in MIS-C need to be elucidated. This review aims to analyze the main results of the recent literature assessing immune response to SARS-CoV-2 over the pediatric age group. We summarized such observations by dividing them into innate and acquired immunity, then reporting how altered immune responses can determine post-infectious conditions.
Impact
-
The main immune markers of acute SARS-CoV-2 infection in children are summarized in this review.
-
This paper reports a broad overview of age-related differences in the immune response to SARS-CoV-2 and emerging post-infection conditions.
-
A summary of currently available therapies for the pediatric age group is provided.
Similar content being viewed by others
Introduction
Since the earliest descriptions of coronavirus disease 2019 (COVID-19), it has become clear that most children experience SARS-CoV-2 infection with a milder clinical course than adults.1,2,3 Severe and critical pediatric cases (2.5% and 0.6%, respectively) are significantly less frequent than in adults even in children with comorbidities.4,5,6,7 Apart from the course of the acute SARS-CoV-2 infection, one of the most remarkable features in pediatrics is the wide range of post-infectious immune and inflammatory conditions including multisystem inflammatory syndrome in children (MIS-C),8,9,10,11,12 cutaneous lesions,13,14,15,16 Long COVID,17 and the recent increased incidence of diabetes.18
Altogether, the evidence of such different clinical pictures in response to SARS-CoV-2 infection suggests the existence of immunological, genetic, viral, or environmental factors that distinguish the “successful” immune response that resists or contains the virus, from the unsuccessful response that leads to the range of severe outcomes.
Understanding the immunological mechanisms underlying the different host responses to SARS-CoV-2 could be critical in identifying individuals at risk of adverse outcomes, preventing progression to severe disease, and improving treatment.
As many of the specificities of COVID-19 clinical pictures are age-related, the immunological response to the virus in children could provide important insights.
Methods
This review aims to analyze the main results of the recent literature assessing immune response to SARS-CoV-2 in children. We attempted a comprehensive search related to COVID-19 in children and major post-infectious conditions by studying the most relevant literature available on PubMed.
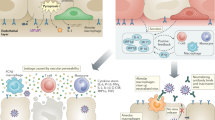
We summarized such observations by dividing them into innate and acquired immunity (Fig. 1), then reporting how altered immune responses can determine post-infectious conditions.
Innate immunity: in healthy children, frequent exposure to viruses and vaccination in the early years of life could keep the immune system ready in case of first exposure to SARS-CoV-2.165 Immediate production of type 1 IFN is decisive in preventing the spread of viral infection, while SARS-CoV-2 escape strategies are frustrated by the intervention of specific lymphocytes that limit its inhibitory anti-IFN activity. In adulthood, the type 1 IFN response is delayed and the increased presence of anti-IFN antibodies favors the spread of infection.46 Infiltration of the lungs by monocytes/macrophages and neutrophil granulocytes, which show a higher presence of adhesion molecules than in children, leads to pneumonia.69 Adaptative immunity: the higher number of naive and regulatory cells leads to a moderate cytokine response in children, avoiding the risk of the cytokine storm typical of more severe COVID-19 cases in adults.75,82 Humoral response: the production of neutralizing antibodies is associated with a better outcome of the SARS-CoV-2 infection. Among adults with severe disease, the mechanism of antibody-dependent enhancement (ADE) has been described.76,77 This would be due to the presence of non-neutralizing antibodies that favor viral propagation and the creation of circulating immune complexes. Created with BioRender.com.
The “barrier” of innate immunity
Virus entry
Once SARS-CoV-2 reaches the human airways via airborne transmission, it infects respiratory cells through binding between the receptor binding domain (RBD) of the spike protein and the receptor of angiotensin-converting enzyme 2 (ACE2) expressed by the respiratory cell membrane.19 TMPRSS2, a transmembrane serine protease, plays a key role in virus entry.20 One of the first hypotheses to explain the difference in clinical manifestations between children and adults is the possible different expression of ACE2 receptors.21,22 Some authors have suggested that ACE2 receptors in children may have a lower affinity for SARS-CoV-2, thus reducing virus entry into the host cell.23 Lower levels of TMPRSS2, have been observed in children and infants.21,24
A recent work revealed that ACE2 protein expression in alveolar type 2 cells of children compared with adults was lower, resulting in higher angiotensin II levels, while TMPRSS2 protein expression was not affected by age.25 On the other hand, several studies showed that there is no clear difference in either viral load or ACE2 expression in the upper airways between children and adults.26,27,28 In conclusion, given the number of supporting studies, it is likely that SARS-CoV-2 binding to respiratory cells is somewhat different and lower in children, but how much this may affect the clinical expression of infection remains controversial.
Recently has been supposed that Omicron (B.1.1.52), a SARS-CoV-2 variant that emerged in late 2021, infects cells independently of the ACE2/TMPRSS2 pathway. In particular, Omicron’s spike protein requires the activity of endosomal cathepsins to be cleaved, while it is unable to efficiently use the cellular protease TMPRSS2.29 This switch of entry strategy increases the number of target cell types for viral entry. Probably this could explain why the Omicron variant is showing higher transmissibility30,31 even in children (Fig. 2).
Regardless of variant, important differences in the expression of molecules involved in the “barrier” of innate immunity have been demonstrated. Epithelial cells lining the upper airways appear to play a crucial role. These cells express pattern recognition receptors, like MDA5 and RIG-I, cytosolic proteins essential for antiviral host immune responses, that can recognize SARS-CoV-2 antigens (Fig. 2). These receptors once recognized double-stranded RNA, trigger activation of TANK-binding kinase 1 (TBK1) through the key mitochondrial antiviral signaling adaptor.32 TBK1 in turn activates interferon regulatory factor 3, which induces the production of type I interferon (IFN-I) and downstream interferon-stimulated genes.33,34 In children, significantly higher expression than in adults of genes encoding MDA5 and RIG-I in upper airway epithelial cells, macrophages, and dendritic cells was detected.35 This massive presence of receptors could optimize the barrier response and immediately trigger interferon production and inflammasome complexing activation including NLR family pyrin domain containing 3. Higher cytokine (IFN-α2, IFN-γ, IP-10, IL-8, and IL-1β) levels found in nasopharyngeal samples from children support this theory.36
The crucial role of interferon
Anti-IFN-I antibodies
A key role in the antiviral response is played by IFN-I through induction of IFN-inducible genes and stimulation of apoptosis in infected cells.26 The activation of this pathway is earlier and better in children due to the massive presence of cytosolic receptors.35 Thus, the difference in the severity of COVID-19 between children and adults could in part be explained by the delay in interferon production in older individuals and the resulting delayed immune response leading to higher viral replication.37
Therefore, as might be expected, the presence of autoantibodies directed against IFN is likely to delay the antiviral response. Indeed, patients with severe COVID-19 show a marked increase in autoantibodies directed against immunomodulatory proteins, including IFN and other cytokines, chemokines, complement, and cell surface proteins. Furthermore, in mouse models of SARS-CoV-2 infection, these autoantibodies have been shown to increase disease severity by promoting higher viral load, altering monocyte activity in the lungs, and reducing the frequency of activated natural killer cells.38 These data confirm the key role of the IFN pathway in the organism’s resistance to SARS-CoV-2.
Interestingly, the expression of anti-IFN-I antibodies has been correlated with age. In a study of people who had not been previously infected with SARS-CoV-2, the prevalence of these antibodies was shown to increase with age.39 In childhood, the increased presence of anti-interferon antibodies is limited to unusual conditions. This is the case of patients with autoimmune polyendocrinopathy syndrome type 1, who frequently express neutralizing autoantibodies directed against IFNs-I, and should be considered at high risk for life-threatening COVID-19 pneumonia.40,41
Other childhood-onset genetic conditions involving a cellular defect and higher levels of these autoantibodies are X-linked immune dysregulation polyendocrinopathy enteropathy,42 and combined T/B cell immunodeficiency due to hypomorphic mutations in RAG1/RAG2.43 However, there are not many reports of severe COVID-19 in these patients, and the most relevant articles on COVID-19 in children with inborn errors of immunity (IEI) do not describe any correlation between the type of IEI and disease severity.41,44,45 To try to understand the discrepancy between the expected severity and what appears to be the actual severity, we need to analyze in deeper detail the subtypes of anti-IFN type 1 autoantibodies.
Bastard et al. found that autoantibodies against IFN-I in patients with severe COVID-19 were directed against all 13 subtypes of IFN-α, while autoantibodies against other IFN-Is (IFN-β, IFN- ω) were rarely present showing a neutralizing capacity in only a small percentage of cases.46 It could be speculated that non-α IFN-I, spared by autoimmunity, may provide adequate compensatory antiviral activity. This might be the reason why not all patients with these genetic conditions are susceptible to severe viral infections, including COVID-19,47,48 but further studies are needed. In any case, these patients may benefit significantly from treatment with neutralizing monoclonal antibodies49 and, in cases of severe evolution, with IFN-beta or plasma exchange.40,50
Predisposing genetic defects
Given the key role of IFN-I in the antiviral response, the presence of pathogenic variants in type I IFN pathway genes could lead to a significant risk of severe COVID-19 in affected children.51,52 Patients with defects in the MDA5-, IRF7- or Toll-like receptors (TLR)-dependent pathways showed altered production of IFN-I, resulting in the risk of severe viral infections.
Plasmacytoid dendritic cells (pDCs) are the most effective producers of IFN-I and play a central role in innate immunity. The pDCs express TLR7 and TLR9 and can sense viral nucleic acids by promoting an antiviral environment. Even in the pre-pandemic era, human X-linked TLR7 deficiency has been described as a determinant of life-threatening influenza in otherwise healthy children.53 Confirming this predisposition, a recent study described a cohort of male patients with COVID-19 pneumonia who have X-linked TLR7 deficiency. In these patients, pDCs show altered IFN-I production after SARS-CoV-2 antigen stimulation.54
Other fragile subjects include individuals with loss-of-function defects in STAT1-, STAT2-, IFNAR1-, IFNAR2-dependent pathways who show altered cellular response to IFN-I and are similarly at risk for severe COVID-19.51,55,56,57,58 Taken together, these results confirm the key role of IFN in response to SARS-CoV-2 and identify a group of patients at high risk of severe SARS-CoV-2 infection.
SARS-CoV-2 mediated IFN-I inhibition
SARS-CoV-2 can counteract and delay the IFN-mediated immune response by approximately 48 h, allowing the virus to replicate and propagate infection.59 Several mechanisms have been proposed to explain this SARS-CoV-2 ability, which involve the degradation of MDA5, RIG-I or TBK1 proteins and the consequently reduced production of IFN-I by cells of the respiratory epithelium and innate immunity.60,61,62 As we will see later, CD4+ T lymphocytes capable of counteracting this viral strategy have been demonstrated in children with SARS-CoV-2 infection.63
The innate lymphoid cells
A particular type of lymphocyte, the innate lymphoid cells (ILCs), may contribute to host resistance toward SARS-CoV-2 infection and promote restoration of tissue damage by producing amphiregulin, a type-II cytokine.64 These cells are known in animal models to ensure tissue integrity and regulate the innate and adaptive immune response with the effect of decreasing disease severity.65 In a recent study, Silverstein et al. showed that the number of ILCs in the blood of patients with SARS-CoV-2 infection was inversely correlated with age and was lower in men, reflecting a higher risk associated with male sex and older age. Interestingly, ILCs were lower in symptomatic children and children with MIS-C compared to healthy controls.66
Neutrophils
During the acute phase of COVID-19, the circulating neutrophils of children are characterized by an activation phenotype due to the presence of specific markers (HLA-DR, CD64, PECAM-1)67 and CD63,68 and an increase in inhibitory receptors (LAIR-1, PDL1). However, the same cells express fewer adhesion molecules.67 For these reasons, it could be assumed that the lung tissue infiltration, which is the basis of lung damage in adults,69 does not occur in the same proportions in children due to a reduced ability of the activated cells to reach deep tissues.
A particular population of neutrophil granulocytes is represented by lower-density granulocytes (LDG). Recent studies show that there is significant recruitment and activation of LDGs in patients with COVID-19. LDGs also appear to have immunosuppressive capacity, which may play a role in the altered lymphocyte response to prolonged infection.70
Adaptative immunity
Significant differences in humoral and cellular immunity have been demonstrated between children and adults, which could contribute to the clinical differences.
Humoral immunity
In the months immediately following the onset of the pandemic, it has been shown that the humoral response against several viral proteins is rapid and occurs in most infected individuals. Some authors pointed to antibodies to common coronaviruses as a possible cause of the better prognosis of COVID-19 in children. Nevertheless, it was observed that the level of antibodies to common coronaviruses and SARS-CoV-2 were similar between children and adults.26,36,71 Moreover, although the level of antibodies to common coronaviruses is amplified in response to SARS-CoV-2, this does not result in any protection from the infection itself.72
The specific response against SARS-CoV-2 is characterized by the almost concomitant appearance of virus-specific IgG, IgA, and IgM neutralizing antibodies (nAbs) directed against several epitopes of the Spike glycoprotein. In particular, antibodies directed against the RBD, i.e., the part of the Spike protein that interacts with the cellular ACE2 receptor,19 can block virus entry and thus the ability to infect cells.
Several studies have investigated the production and neutralizing capacity of anti-SARS-CoV-2 immunoglobulins.73,74,75 However, a distinction must be made between nAbs, which are essential in the response against SARS-CoV-2, and non-specific antibodies. The latter, according to some authors, may even be detrimental since they can mediate antibody-dependent enhancement (ADE), a key mechanism in the pathogenesis of COVID-1976,77 (Fig. 1).
In early 2021, a work published by Yang et al. showed that SARS-CoV-2-specific antibody response profiles are distinct in different age groups, with children showing higher median levels of IgG.78 Further works strengthened these data79,80 observing that the nAbs titer was inversely correlated with age, with children under 6 years of age showing the highest titers at onset and subsequent determinations. Also, nAbs against SARS-CoV-2 persisted for a period of 2–8 months after infection.79
Increased levels of cross-reactive but non-neutralizing IgG against SARS-CoV-2 were detected in pre-pandemic samples of healthy elderly people. In the same study, healthy children showed elevated coronaviruses-specific IgM. This suggests that children have less exposure to human coronaviruses, resulting in a less experienced but more polyreactive humoral immunity.80 Remarkably, children with higher levels of anti-SARS-CoV-2 IgG and higher concentrations of nAbs show lower viral load and faster virus clearance.12 This information further confirms that pediatric patients can mount a humoral response reasonably soon after the onset of symptoms.81
A proteomic analysis revealed that some molecules involved in the lymphocyte activation pathway (SLAMF1 and CD244), in response to antigens processed by the MHC, were significantly higher in children who produced nAbs than those with antibodies without neutralizing activity.82 These findings could link to the concept of trained immunity, i.e., an increased basal tone of innate immunity in response to certain vaccines and microbial components, which could contribute to resistance against infection.83,84 The higher frequency of infections and vaccinations in childhood could thus “train” the immune system and partly explain the readiness in response to SARS-CoV-2.85 Indeed, some authors have proposed that measles, mumps and rubella vaccines as well as Bacillus Calmette–Guerin may provide protection from severe COVID-19 and various clinical trials are still ongoing.86,87,88,89 The presence of a homologous sequence between measles, rubella and SARS-CoV-2 spike protein could strengthen this theory.90,91 Thus, innate immunity would play an important role in the stimulation of anti-SARS-CoV-2 nAbs response.
On the other hand, the level of specific response against SARS-CoV-2 does not seem to be related to the severity of the clinical picture. Both asymptomatic and symptomatic children can develop SARS-CoV-2-specific adaptive immunity at a similar level in terms of total Ab for SARS-CoV-2, Ab-mediated neutralization, and Ag-specific B cells and CD8 T cells.92
In conclusion, the neutralizing humoral response is more ready and effective in children, whereas in older individuals, a propensity to trigger the counterproductive mechanism of ADE has been demonstrated.77
Cellular immunity
The high invasiveness of SARS-CoV-2 challenges adaptive cellular immunity. Continued infection forces the host to deplete its natural killer and T cells, resulting in lymphopenia.93 The inability to eradicate the infection causes the abnormal release of excessive inflammatory cytokines to compensate for lymphocyte depletion; this pathway leads to cytokine storm syndrome.
Indeed, lymphopenia is recognized as a marker of severity of the clinical picture,26,94 but in childhood is rarely observed.95 It was found that children hospitalized for COVID-19 had a higher value of lymphocytes than adults36,95 who, however, show higher levels of specific T lymphocytes.96,97 Recently, it has been shown that low frequencies in naive CD8+ and CD4+ T cells correlate with age, and COVID-19 disease severity.98
On the other hand, the immunological memory of T cells, could play a role in the pathophysiology and subsequent collateral tissue damage in patients with COVID-19.99 It has long been known that the T-cell response changes with age. The expression of CD45RA, a typical marker of naïve cells, decreases linearly with age, and circulating memory cells outnumber naïve cells at approximately 35 years of age.100 Interestingly, in vitro-induced toxic shock syndrome has been demonstrated to be less severe among children because of a difference in T-cell response101 with the higher number of CD45RA proposed as a protective factor. Memory T cells in patients recovered from COVID-19 showed significantly higher amplitude and magnitude responses in severe cases compared with mild cases, using IFN-γ-based assays.
Cellular B memory also shows the same trend with respect to the severity of infection and age. Low levels of memory B cells (IgD-CD27+) correlate with an asymptomatic or mild infection in children.102
Remarkably, the TCR specificities of CD4+ T cells vary between children and adults. In contrast to adult T cells specific mainly for structural proteins of SARS-CoV-2, a recent study shows that CD4+ T cells from children with COVID-19 were more specific for open reading frame 1ab (Orf1ab), which encodes for non-structural proteins that are responsible for inhibition of IFN production by the host.63,103 These findings seem to confirm, once again, the key role of the age-associated IFN response.
Regulatory cells represent another factor that could explain the more effective containment of the virus by children. Indeed, both Tregs and Bregs cells were found in higher amounts in children with COVID-19 than in adults and also correlated positively with the level of nAbs.104 FoxP3+/CD25high Tregs cells are also increased in pediatric cases with moderate-to-severe clinical course compared to infected adults.102
In children, the lymphocyte response to SARS-CoV-2 is characterized by a lower amount of pro-inflammatory cytokines than in adults, being more skewed toward a Th2 response.36,105 This could partly explain the higher inflammatory cascade in the adult population and the consequent higher severity of the disease. In severe and fatal cases of COVID-19, elevated innate immune cytokines were detected in peripheral blood, including IL-1, IL-6, IL-8, or C-X-C Motif Chemokine Ligand 10.37,106,107 Pierce et al. described significant differences in cytokines of 65 children and youth (<24 years) compared with adults with SARS-CoV-2 regardless of the severity of symptoms. Specifically, serum concentrations of interleukin-17A (IL-17A) and IFN-γ were inversely correlated with age.36 The authors suggest that the early immune response, evidenced by increased IL-17A and IFN- γ, leads in children to a more rapid resolution of viral infection.
However, when the triggered inflammatory response is critical, as in children with severe COVID-19 or MIS-C, an increase in IL-1, IL-2, IL-6, IL-10, IL-13, and IL-17 has been described compared to children with mild/moderate disease.12,108,109,110
Post-COVID-19 conditions
Compared to other common respiratory infections, SARS-CoV-2 induces a greater and longer-lasting cellular response that persists for months, even in mild or asymptomatic forms. In a recent work, polyclonal stimulation resulted in significantly greater activation of T cells in individuals who had recently experienced mild SARS-CoV-2 infection, compared with individuals with other recent respiratory infections.111 This peculiar characteristic could partially explain the high frequency of inflammatory conditions weeks after the SARS-CoV-2 infection, such as MIS-C and MIS-A. In recent months, anti-SARS-CoV-2 post-vaccine reactions, including MIS-C-like forms,112,113,114 myocarditis,115,116,117 or autoimmune disorders have been described.118 However, the incidence of adverse events following immunization is rare and not comparable with post-infection complications even in children.119
MIS-C/A
MIS-C, also known as pediatric multisystemic inflammatory syndrome, occurs 2–5 weeks after SARS-CoV-2 infection and presents as a spectrum of inflammatory diseases with persistent fever, elevated inflammatory markers, rash, conjunctival injection, and progressing in severe cases to shock, with impairment of myocardial function and multi-organ involvement. MIS-C, which meets the WHO definition of the disorder, is probably the “tip of the iceberg” of a broader spectrum of inflammatory diseases that occur after SARS-CoV-2 infection. MIS-C is a common cause of admission to hospitals around the world and the estimated incidence surpasses the frequency of severe COVID-19 in children.120,121,122,123 The disorder presents considerable diagnostic difficulties, as the clinical features overlap with those of bacterial sepsis, Kawasaki disease (KD), and other inflammatory diseases.9,124 Interestingly, early data on the comparison of MIS-C cases between the first three pandemic waves showed overall improvement in outcomes including the severity of cardiovascular complications.125
MIS-C shows remarkable clinical similarities with KD shock syndrome rather than classic or atypical KD. Patients with MIS-C show higher values of inflammation, and a tendency to lymphopenia and thrombocytopenia rather than the thrombocytosis observed in classic KD.8 Also, age seems to play a role since MIS-C cases have a higher mean age than KD (8–9 vs 3 years).126 The results of an international survey revealed that patients with a KD-like phenotype show a lower mean age127 without shock signs and fewer gastrointestinal, cardiorespiratory, and neurologic symptoms. However, no clear differences in clinical severity between age groups are described in patients with MIS-C.128 Another distinguishing feature is cardiac involvement more often represented by reduced ventricular function rather than coronary artery vasculitis, which usually occurs in KD. Coronary involvement is also frequent in MIS-C but at a very early stage.9 The timing of the disorder, occurring weeks after SARS-CoV-2 infection in individuals who have generally recovered from a mild or asymptomatic primary infection, suggests that MIS-C is mediated by an abnormal acquired immune response. The growing literature on MIS-C indicates that the disorder is associated with high levels of antibodies to SARS-CoV-2, augmented levels of Th17 and Th1 cells, and an intense cytokine response involving INFγ, IL-17, with neutrophil activation.10,12,109,129,130,131
Interestingly, the correlation between CD8+ T-cell activation and cardiac dysfunction parameters such as BNP and troponin has recently been described.132 Genetically based alterations in Treg cells have also been proposed for the pathogenesis of MIS-C. Indeed, a recent study showed in patients with MIS-C deleterious variants that lead to upregulation of Notch1 in Treg cells.133 This mechanism could underlie the Th1-skewed systemic inflammation.
Since MIS-C has a peculiar geographical distribution with few cases described among East Asian populations,134 a predisposing genetic substrate can be assumed. Like the monogenic defects involving innate immunity and IFN1-mediated response that can lead to the risk of severe COVID-19 pneumonia,46,51,52 constitutional disorders involving the adaptive immune response can also be thought for MIS-C. Several genetic defects have already been proposed as risk factors for severe forms of MIS-C,131,135,136,137 but further studies on large case series could lead to a clearer understanding of the pathogenesis of this condition.
Finally, the superantigen theory has been proposed. This is based on the presence of a superantigen-like motif within the S protein of SARS-CoV-2.138 It has been suggested that the inflammatory cascade of MIS-C is due in part to the ability of this domain to bind with high affinity to the α chain and β chain variable region of the TCR. Interestingly, this domain has a remarkable structural similarity to the staphylococcal enterotoxin B (SEB) superantigen. It has been proposed that some children may be less susceptible to the development of MIS-C due to the prior development of antibodies directed against SEB.138 Because first-line therapy of MIS-C includes treatment with immunoglobulins, it is possible that its efficacy is in part due to anti-SEB antibodies present in IVIG preparations.139
A post-SARS-CoV-2 condition very similar to MIS-C has been observed in adults. MIS-A is defined as an inflammatory syndrome with severe extrapulmonary organ dysfunction, usually affecting persons over 21 years of age with laboratory evidence of previous SARS-CoV-2 infection (within 12 weeks), and absence of severe respiratory disease.140,141,142 Impaired production of IFN-I and IFN-III has also been described in this illness.143
MIS-V
Recently, a multisystem inflammatory syndrome temporally associated with SARS-CoV-2 vaccination (MIS-V) has been described.112,113,114 As for MIS-C, MIS-V is also an almost exclusively pediatric form, as cases reported in the literature among adults are anecdotal.144 Early reports have proposed that individuals with MIS-V manifest immune system hyperresponsiveness due to recent asymptomatic or symptomatic SARS-CoV-2 infection often suggested by a familial cluster or ascertained by nasopharyngeal swab.145,146 Hence, the vaccination could be a trigger of inflammation in individuals who were recently infected.
More recently, Ouldali et al. described in a cohort of 4,079,234 children (aged 12–17 years) the occurrence of MIS-V within days of the vaccine in 12 children, confirming its rarity. Interestingly, 10/12 were male. In 8 of these, recent infection was ruled out by anti-N antibody negativity advancing the idea that the condition is solely induced by vaccination.119 However, comparing the rate of MIS-C and MIS-V in the same age group and population (113 vs. 2.9 per 1,000,000, respectively119), it remains recommended to still use vaccine prophylaxis if the virus is widely spread. In addition, recent studies confirm that the SARS-CoV-2 mRNA vaccine can significantly reduce the incidence of MIS-C.147,148
Long COVID or PASC
The term “long COVID” or “post-acute severe SARS-CoV-2 sequelae” (PASC) refers to the persistence of symptoms, such as fatigue, dyspnea, sleep disturbances, and depression, for more than 3 months after acute infection.149 It has been observed mainly in young people aged 12 years or older. The pediatric age group is also affected by long COVID but the persistence of symptoms in children rarely continues beyond 8 weeks.150
A recent article demonstrates elevated levels of IFN-I and IFN-III and other cytokines 4 months after infection in patients with previous COVID-19 compared to patients with common coronavirus infection.151 In addition, some studies have compared adult patients with long COVID with infected individuals without long COVID, showing reduced cortisol levels, reduced numbers of dendritic cells and exhausted T lymphocytes,152 and increased levels of activated cells, IL-17 and IL-2.153 These data suggest that a persistent immune-mediated inflammatory response underlies the long COVID syndrome. The reasons for this persistent immune activation remain to be elucidated.
Interestingly, some authors have proposed a role for autoimmunity in the pathogenesis of PASC.154 It has been described that about 44% of patients after 1 year from the onset of COVID-19 symptoms developed antinuclear antibody (ANA) titers ≥1:160. In this group, the frequency of neurocognitive symptoms was significantly higher than in ANA-negative subjects.155 However, the most significant studies in PASC concern adult patients.156 Further studies are needed in this regard, especially in adolescent age. Recently, Brodin et al. studied patients with long COVID suggesting various mechanisms that could result in long COVID occurring, including viral persistence, SARS-CoV-2 superantigen-mediated activation of the immune system, and autoimmunity.157
Diabetes
Recently, there has been a growing concern after the observation of a significant increase in new diagnoses of diabetes mellitus after SARS-CoV-2 infection in children (<18 years).18 This increased incidence was significant in patients newly infected with SARS-CoV-2, compared to those not exposed to the virus and those with other respiratory infections. Although enhanced expression of ACE2 has been demonstrated in pancreatic beta cells compared to the lung, it is still unclear whether there is direct damage caused by SARS-COV-2 on Langerhans’ Islets.158,159 Various factors are likely to play a role. These include hyperglycemia induced during lockdown by sedentariness and dietary changes and the pro-inflammatory state that may involve the pancreas at the end of the cytokine cascade.158
Severe acute hepatitis of unknown etiology
In the early months of 2022, considerable concern has arisen over several cases of severe acute hepatitis of unknown etiology in children. As of May 13, 2022, the reported cases in children aged 16 years or below were 232.160 Adenovirus 41F has been proposed as an agent for these forms since it has been found in more than 70% of cases reported in the UK.161 A clear link to SARS-CoV-2 has not yet been found, but in some cases, children tested positive for SARS-CoV-2 on admission, and 11/12 patients in the cohort described in Israel had COVID-19 in the previous months.162 A recent letter from Brodin and Arditi suggests that the SARS-CoV-2 persistence in the gastrointestinal tract due to the barrier abnormalities already described in MIS-C patients163 may represent a predisposing substrate. Adenovirus 41F could thus be a second trigger for the development of acute hepatitis, probably through a superantigen-mediated immune response.164 Interestingly, the average age of children with this hepatitis appears to be very low, thus being an unvaccinated SARS-CoV-2 population.
Conclusions
A remarkable feature of the COVID-19 pandemic is the wide range of outcomes following SARS-CoV-2 infection and the different outcomes in children and adults. While a large proportion of the population has an asymptomatic and mild illness, others suffer severe or fatal disease and post-infectious complications including post-COVID-19 sequalae and multisystem inflammatory syndromes. Answers to the key questions posed by the widely differing responses to SARS-CoV-2 infection need to be searched in the uniqueness of children’s immune system. Future studies based on the comparison of the host response to SARS-CoV-2 in children and adults of increasing age through system biology approach could provide an important opportunity to understand how the “successful immune response” differs from the “unsuccessful or disease enhancing response” seen in the elderly as well as in pediatric post-infection conditions.
Data availability
The data are available on request by contacting the corresponding author.
References
Bai, K. et al. Clinical analysis of 25 COVID-19 infections in children. Pediatr. Infect. Dis. J. 39, e100–e103 (2020).
Dong, Y. et al. Epidemiology of COVID-19 among children in China. Pediatrics 145, e20200702 (2020).
Wang, Y. et al. Children hospitalized with severe COVID-19 in Wuhan. Pediatr. Infect. Dis. J. 39, e91–e94 (2020).
Onder, G., Rezza, G. & Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323, 1775–1776 (2020).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180, 934–943 (2020).
Brisca, G. et al. The impact of COVID-19 lockdown on children with medical complexity in pediatric emergency department. Am. J. Emerg. Med. 42, 225–227 (2021).
Bellini, T. et al. Characteristics of COVID-19 patients up to 6 months of age admitted to a paediatric emergency department. Acta Paediatr. 111, 272–274. https://doi.org/10.1111/apa.16166 (2022).
Cattalini, M. et al. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): a diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital. J. Pediatr. 47, 24 (2021).
Feldstein, L. R. et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325, 1074–1087. https://doi.org/10.1001/jama.2021.2091 (2021).
Gruber, C. N. et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 183, 982–995.e14 (2020).
Whittaker, E. et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324, 259–269 (2020).
Consiglio, C. R. et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 183, 968–981.e7 (2020).
Rotulo, G. A. et al. Giant urticaria and acral peeling in a child with coronavirus disease 2019. J. Pediatr. 230, 261–263 (2021).
Signa, S. et al. Recurrence of previous chilblain lesions during the second wave of COVID-19: can we still doubt the correlation with SARS-CoV-2? J. Eur. Acad. Dermatol. Venereol. 35, e475–e477 (2021).
Kanitakis, J., Lesort, C., Danset, M. & Jullien, D. Chilblain-like acral lesions during the COVID-19 pandemic (‘COVID toes’): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J. Am. Acad. Dermatol. 83, 870–875 (2020).
Lesort, C. et al. COVID-19 and outbreak of chilblains: are they related? J. Eur. Acad. Dermatol. Venereol. 34, e757–e758 (2020).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Lond. Engl. 397, 220–232 (2021).
Barrett, C. E. et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years—United States, March 1, 2020–June 28, 2021. MMWR Morb. Mortal. Wkly. Rep. 71, 59–65 (2022).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020).
Lan, J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020).
Sharif-Askari, N. S. et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol. Ther. Methods Clin. Dev. 18, 1–6 (2020).
Zhang, Z. et al. Distinct disease severity between children and older adults with coronavirus disease 2019 (COVID-19): impacts of ACE2 expression, distribution, and lung progenitor cells. Clin. Infect. Dis. 73, e4154–e4165 (2021).
Zimmermann, P. & Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 106, 429–439 (2021).
Schuler, B. A. et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Invest 131, e140766 (2021).
Silva, M. G. et al. Effect of age on human ACE2 and ACE2-expressing alveolar type II cells levels. Pediatr. Res. 1–5. https://doi.org/10.1038/s41390-022-02163-z (2022).
Pierce, C. A. et al. Natural mucosal barriers and COVID-19 in children. JCI Insight 6, e148694 (2021).
Bunyavanich, S., Do, A. & Vicencio, A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323, 2427 (2020).
Heald-Sargent, T. et al. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatr. 174, 902 (2020).
Zhao, H. et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg. Microbes Infect. 11, 277–283 (2022).
Bálint, G., Vörös-Horváth, B. & Széchenyi, A. Omicron: increased transmissibility and decreased pathogenicity. Signal Transduct. Target. Ther. 7, 1–3 (2022).
Araf, Y. et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 94, 1825–1832 (2022).
Seth, R. B., Sun, L., Ea, C.-K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005).
Chiu, Y.-H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Yoo, J.-S., Kato, H. & Fujita, T. Sensing viral invasion by RIG-I like receptors. Curr. Opin. Microbiol. 20, 131–138 (2014).
Loske, J. et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 40, 319–324. https://doi.org/10.1038/s41587-021-01037-9 (2022).
Pierce, C. A. et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 12, eabd5487 (2020).
Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e9 (2020).
Wang, E. Y. et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021).
Bastard, P. et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci. Immunol. 6, eabl4340 (2021).
Lemarquis, A. et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy Clin. Immunol. 148, 96–98 (2021).
Meyts, I. et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J. Allergy Clin. Immunol. 147, 520–531 (2021).
Rosenberg, J. M. et al. Neutralizing anti-cytokine autoantibodies against interferon-α in immunodysregulation polyendocrinopathy enteropathy X-linked. Front. Immunol. 9, 544 (2018).
Walter, J. E. et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest 125, 4135–4148 (2015).
Bucciol, G., Tangye, S. G. & Meyts, I. Coronavirus disease 2019 in patients with inborn errors of immunity: lessons learned. Curr. Opin. Pediatr. 33, 648–656 (2021).
Meisel, C. et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Invest. 131, e150867 (2021).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020).
Meager, A. et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 3, e289 (2006).
Milito, C. et al. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J. Allergy Clin. Immunol. Pract. 9, 2904–2906.e2 (2021).
Ferré, E. M. N. et al. SARS-CoV-2 spike protein-directed monoclonal antibodies may ameliorate COVID-19 complications in APECED patients. Front. Immunol. 12, 720205 (2021).
Kakuturu, J., McCluskey, C., Casey, F. L., Cicek, S. & Hayanga, J. W. A. Extracorporeal membrane oxygenation to treat a 15-year-old patient with severe coronavirus disease 2019 (COVID-19) respiratory failure. JTCVS Tech. 7, 265–266 (2021).
Zhang, Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020).
Zhang, Q., Bastard, P., Cobat, A. & Casanova, J.-L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 603, 587–598. https://doi.org/10.1038/s41586-022-04447-0 (2022).
Ciancanelli, M. J. et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 348, 448–453 (2015).
Asano, T. et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6, eabl4348 (2021).
Matsuyama, T., Kubli, S. P., Yoshinaga, S. K., Pfeffer, K. & Mak, T. W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 27, 3209–3225 (2020).
Abolhassani, H. et al. Inherited IFNAR1 deficiency in a child with both critical COVID-19 pneumonia and multisystem inflammatory syndrome. J. Clin. Immunol. 42, 471–483. https://doi.org/10.1007/s10875-022-01215-7 (2022).
Khanmohammadi, S., Rezaei, N., Khazaei, M. & Shirkani, A. A case of autosomal recessive interferon alpha/beta receptor alpha chain (IFNAR1) deficiency with severe COVID-19. J. Clin. Immunol. 42, 19–24 (2022).
Schulert, G. S., Blum, S. A. & Cron, R. Q. Host genetics of pediatric SARS-CoV-2 COVID-19 and multisystem inflammatory syndrome in children. Curr. Opin. Pediatr. 33, 549–555 (2021).
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374 (2020).
Sui, L. et al. SARS-CoV-2 membrane protein inhibits type i interferon production through ubiquitin-mediated degradation of TBK1. Front. Immunol. 12, 662989 (2021).
Fu, Y.-Z. et al. SARS-CoV-2 membrane glycoprotein M antagonizes the MAVS-mediated innate antiviral response. Cell. Mol. Immunol. 18, 613–620 (2021).
Zheng, Y. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct. Target. Ther. 5, 299 (2020).
Xia, H. & Shi, P.-Y. Antagonism of type i interferon by severe acute respiratory syndrome coronavirus 2. J. Interferon Cytokine Res. 40, 543–548 (2020).
Singh, S. S. et al. Amphiregulin in cellular physiology, health, and disease: potential use as a biomarker and therapeutic target. J. Cell. Physiol. 237, 1143–1156 (2022).
Diefenbach, A., Gnafakis, S. & Shomrat, O. Innate lymphoid cell-epithelial cell modules sustain intestinal homeostasis. Immunity 52, 452–463 (2020).
Silverstein, N. J. et al. Innate lymphoid cells and COVID-19 severity in SARS-CoV-2 infection. eLife 11, e74681 (2022).
Seery, V. et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 67, 103357 (2021).
Neeland, M. R. et al. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat. Commun. 12, 1084 (2021).
Tomar, B., Anders, H.-J., Desai, J. & Mulay, S. R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells 9, E1383 (2020).
Cabrera, L. E. et al. Characterization of low-density granulocytes in COVID-19. PLoS Pathog. 17, e1009721 (2021).
Di Chiara, C. et al. Long-term immune response to SARS-CoV-2 infection among children and adults after mild infection. JAMA Netw. Open 5, e2221616 (2022).
Anderson, E. M. et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 184, 1858–1864.e10 (2021).
Zhao, J. et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 71, 2027–2034 (2020).
Long, Q.-X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020).
Ju, B. et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584, 115–119 (2020).
Wan, Y. et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 94, e02015–e02019 (2020).
Lee, W. S., Wheatley, A. K., Kent, S. J. & DeKosky, B. J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 5, 1185–1191 (2020).
Yang, H. S. et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw. Open 4, e214302 (2021).
Bonfante, F. et al. Mild SARS-CoV-2 infections and neutralizing antibody titers. Pediatrics 148, e2021052173 (2021).
Selva, K. J. et al. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 12, 2037 (2021).
Yonker, L. M. et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J. Pediatr. 227, 45–52.e5 (2020).
Cotugno, N. et al. Virological and immunological features of SARS-CoV-2-infected children who develop neutralizing antibodies. Cell Rep. 34, 108852 (2021).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
de Laval, B. et al. C/EBPβ-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell 26, 793 (2020).
Mantovani, A. & Netea, M. G. Trained innate immunity, epigenetics, and COVID-19. N. Engl. J. Med. 383, 1078–1080 (2020).
Brueggeman, J. M., Zhao, J., Schank, M., Yao, Z. Q. & Moorman, J. P. Trained immunity: an overview and the impact on COVID-19. Front. Immunol. 13, 837524 (2022).
O’Neill, L. A. J. & Netea, M. G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 20, 335–337 (2020).
Hajjo, R. & Tropsha, A. A systems biology workflow for drug and vaccine repurposing: identifying small-molecule BCG mimics to reduce or prevent COVID-19 mortality. Pharm. Res. 37, 212 (2020).
Murdoch Childrens Research Institute. BCG Vaccination to Reduce the Impact of COVID-19 in Healthcare Workers (BRACE) Trial; https://clinicaltrials.gov/ct2/show/NCT04327206 (2022).
Sidiq, K. R., Sabir, D. K., Ali, S. M. & Kodzius, R. Does early childhood vaccination protect against COVID-19? Front. Mol. Biosci. 7, 120 (2020).
Walls, A. C. et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292.e6 (2020).
Cotugno, N. et al. Virological and immunological features of SARS‐COV‐2 infected children with distinct symptomatology. Pediatr. Allergy Immunol. 32, 1833–1842 (2021).
Fathi, N. & Rezaei, N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol. Int. 44, 1792–1797 (2020).
Kosmeri, C., Koumpis, E., Tsabouri, S., Siomou, E. & Makis, A. Hematological manifestations of SARS-CoV-2 in children. Pediatr. Blood Cancer 67, e28745 (2020).
Ma, X. et al. The clinical characteristics of pediatric inpatients with SARS-CoV-2 infection: a meta-analysis and systematic review. J. Med. Virol. 93, 234–240 (2021).
Cohen, C. A. et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 12, 4678 (2021).
Kaaijk, P. et al. Children and adults with mild COVID-19: dynamics of the memory T Cell response up to 10 months. Front. Immunol. 13, 817876 (2022).
Rydyznski Moderbacher, C. et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183, 996–1012.e19 (2020).
de Candia, P., Prattichizzo, F., Garavelli, S. & Matarese, G. T cells: warriors of SARS-CoV-2 infection. Trends Immunol. 42, 18–30 (2021).
Shearer, W. T. et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 112, 973–980 (2003).
Rudolph, M. E. et al. Differences between pediatric and adult T Cell responses to in vitro staphylococcal enterotoxin B stimulation. Front. Immunol. 9, 498 (2018).
Di Sante, G. et al. Immunopathology of SARS-CoV-2 infection: a focus on T regulatory and B cell responses in children compared with adults. Children (Basel) 9, 681 (2022).
Zheng, M. et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 22, 829–838 (2021).
Petrara, M. R. et al. Asymptomatic and mild SARS-CoV-2 infections elicit lower immune activation and higher specific neutralizing antibodies in children than in adults. Front. Immunol. 12, 741796 (2021).
Dowling, D. J. & Levy, O. Ontogeny of early life immunity. Trends Immunol. 35, 299–310 (2014).
Laing, A. G. et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 26, 1623–1635 (2020).
Del Valle, D. M. et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26, 1636–1643 (2020).
Lee, P. Y. et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. J. Clin. Invest. 130, 5942–5950 (2020).
Ravichandran, S. et al. SARS-CoV-2 immune repertoire in MIS-C and pediatric COVID-19. Nat. Immunol. 22, 1452–1464 (2021).
Rodrigues, T. S. et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 218, e20201707 (2021).
Kennedy, A. E. et al. Lasting changes to circulating leukocytes in people with mild SARS-CoV-2 infections. Viruses 13, 2239 (2021).
Yousaf, A. R. et al. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc. Health 6, 303–312 (2022).
Karatzios, C. et al. Multisystem inflammatory syndrome following SARS-CoV-2 vaccination in two children. Pediatrics 150, e2021055956 (2022).
Santilli, V. et al. Two pediatric cases of multisystem inflammatory syndrome with overlapping neurological involvement following SARS-CoV-2 vaccination and unknown SARS-CoV2 infection: the importance of pre-vaccination history. Vaccines 10, 1136 (2022).
Oster, M. E. et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US From December 2020 to August 2021. JAMA 327, 331–340 (2022).
Wong, H.-L. et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet Lond. Engl. 399, 2191–2199 (2022).
Manno, E. C. et al. Higher troponin levels on admission are associated with persistent cardiac magnetic resonance lesions in children developing myocarditis after mRNA-based COVID-19 vaccination. Pediatr. Infect. Dis. J. 42, 166–171. https://doi.org/10.1097/INF.0000000000003762 (2023).
Chen, Y. et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 165, 386–401 (2022).
Ouldali, N. et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg. Health Eur. 17, 100393 (2022).
Levy, N. et al. Severity and incidence of multisystem inflammatory syndrome in children during 3 SARS-CoV-2 pandemic waves in Israel. JAMA 327, 2452–2454 (2022).
Smith, C. et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat. Med. 28, 185–192 (2022).
Kompaniyets, L. et al. Post-COVID-19 symptoms and conditions among children and adolescents – United States, March 1, 2020-January 31, 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 993–999 (2022).
Payne, A. B. et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw. Open 4, e2116420 (2021).
Dufort, E. M. et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 383, 347–358. https://doi.org/10.1056/NEJMoa2021756 (2020).
Miller, A. D. et al. Multisystem inflammatory syndrome in children-United States, February 2020-July 2021. Clin. Infect. Dis. 75, e1165–e1175 (2022).
Abrams, J. Y. et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J. Pediatr. 226, 45–54.e1 (2020).
Bautista-Rodriguez, C. et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics 147, e2020024554 (2021).
Alkan, G., Sert, A., Oz, S. K. T., Emiroglu, M. & Yılmaz, R. Clinical features and outcome of MIS-C patients: an experience from Central Anatolia. Clin. Rheumatol. 40, 4179–4189 (2021).
Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 27, 28–33 (2021).
de Cevins, C. et al. A monocyte/dendritic cell molecular signature of SARS-CoV-2-related multisystem inflammatory syndrome in children with severe myocarditis. Med 2, 1072–1092.e7 (2021).
Lee, P. Y. et al. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J. Allergy Clin. Immunol. 146, 1194–1200.e1 (2020).
Kumar, D. et al. Distinguishing immune activation and inflammatory signatures of multisystem inflammatory syndrome in children (MIS-C) versus hemophagocytic lymphohistiocytosis. J. Allergy Clin. Immunol. 149, 1592–1606.e16 (2022).
Benamar, M. et al. The Notch1/CD22 signaling axis disrupts Treg cell function in SARS-CoV2-associated multisystem inflammatory syndrome in children. J. Clin. Invest. e163235. https://doi.org/10.1172/JCI163235 (2022).
Choe, Y. J. et al. Surveillance of COVID-19–associated multisystem inflammatory syndrome in children, South Korea. Emerg. Infect. Dis. 27, 1196–1200 (2021).
Chou, J. et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J. Allergy Clin. Immunol. 148, 732–738.e1 (2021).
Lee, D. et al. Inborn errors of OAS-RNase L in SARS-CoV-2-related multisystem inflammatory syndrome in children. Science 379, eabo3627 (2023).
Brodin, P. Exaggerated responses to a virus long gone. Science 379, 538–539 (2023).
Cheng, M. H. et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl Acad. Sci. USA. 117, 25254–25262 (2020).
Martinez, O. M., Bridges, N. D., Goldmuntz, E. & Pascual, V. The immune roadmap for understanding multi-system inflammatory syndrome in children: opportunities and challenges. Nat. Med. 26, 1819–1824 (2020).
Parums, D. V. Editorial: multisystem inflammatory syndrome in adults (MIS-A) and the spectrum of COVID-19. Med. Sci. Monit. 27, e935005 (2021).
Weatherhead, J. E., Clark, E., Vogel, T. P., Atmar, R. L. & Kulkarni, P. A. Inflammatory syndromes associated with SARS-CoV-2 infection: dysregulation of the immune response across the age spectrum. J. Clin. Invest 130, 6194–6197 (2020).
Morris, S. B. et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1450–1456 (2020).
Ronit, A. et al. Host genetics and antiviral immune responses in adult patients with multisystem inflammatory syndrome. Front. Immunol. 12, 718744 (2021).
Brown, M. et al. A case of adult multisystem inflammatory syndrome following COVID-19 vaccine. J. Community Hosp. Intern. Med. Perspect. 12, 7–13 (2022).
Buchhorn, R., Meyer, C., Schulze-Forster, K., Junker, J. & Heidecke, H. Autoantibody release in children after corona virus mRNA vaccination: a risk factor of multisystem inflammatory syndrome? Vaccines 9, 1353 (2021).
Salzman, M. B., Huang, C.-W., O’Brien, C. M. & Castillo, R. D. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg. Infect. Dis. 27, 1944–1948 (2021).
Levy, M. et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA 327, 281–283 (2022).
Zambrano, L. D. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years — United States, July–December 2021. MMWR Morb. Mortal. Wkly. Rep. 71, 52–58 (2022).
Buonsenso, D. et al. Preliminary evidence on long COVID in children. Acta Paediatr. 110, 2208–2211 (2021).
Zimmermann, P., Pittet, L. F. & Curtis, N. How common is long COVID in children and adolescents? Pediatr. Infect. Dis. J. 40, e482–e487 (2021).
Phetsouphanh, C. et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 23, 210–216 (2022).
Klein, J. et al. Distinguishing features of Long COVID identified through immune profiling. Preprint at medRxiv https://doi.org/10.1101/2022.08.09.22278592 (2022).
Queiroz, M. A. F. et al. Cytokine profiles associated with acute COVID-19 and Long COVID-19 syndrome. Front. Cell. Infect. Microbiol. 12, 922422 (2022).
Knight, J. S. et al. The intersection of COVID-19 and autoimmunity. J. Clin. Invest. 131, e154886 (2021).
Seeßle, J. et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin. Infect. Dis. 74, 1191–1198. https://doi.org/10.1093/cid/ciab611 (2022).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895.e20 (2022).
Brodin, P. et al. Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat. Med. 28, 879–882. https://doi.org/10.1038/s41591-022-01766-7 (2022).
Liu, F. et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 18, 2128–2130.e2 (2020).
Fignani, D. et al. SARS-CoV-2 Receptor Angiotensin I-Converting Enzyme Type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front. Endocrinol. 11, 596898 (2020).
Increase in severe acute hepatitis cases of unknown aetiology in children. European Centre for Disease Prevention and Control; https://www.ecdc.europa.eu/en/increase-severe-acute-hepatitis-cases-unknown-aetiology-children.
Acute hepatitis: technical briefing. GOV.UK; https://www.gov.uk/government/publications/acute-hepatitis-technical-briefing.
Israel examining 12 cases of kids’ hepatitis after WHO warning – Israel News. https://www.haaretz.com/israel-news/israel-examining-12-cases-of-kids-hepatitis-after-who-warning-1.10752779.
Yonker, L. M. et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Invest. 131, 149633 (2021).
Brodin, P. & Arditi, M. Severe acute hepatitis in children: investigate SARS-CoV-2 superantigens. Lancet Gastroenterol. Hepatol. 7, 594–595. https://doi.org/10.1016/S2468-1253(22)00166-2 (2022).
Yang, F. et al. Shared B cell memory to coronaviruses and other pathogens varies in human age groups and tissues. Science 372, 738–741 (2021).
Children. COVID-19 Treatment Guidelines; https://www.covid19treatmentguidelines.nih.gov/special-populations/children/.
Author information
Authors and Affiliations
Contributions
G.A.R. performed the literature review and draft of the initial version of the manuscript, including figures. P.P. conceived the work, critically reviewed, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rotulo, G.A., Palma, P. Understanding COVID-19 in children: immune determinants and post-infection conditions. Pediatr Res 94, 434–442 (2023). https://doi.org/10.1038/s41390-023-02549-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02549-7
This article is cited by
-
Effectiveness of COVID-19 vaccines among children 6–11 years against hospitalization during Omicron predominance in Malaysia
Scientific Reports (2024)
-
Delineating immune variation between adult and children COVID-19 cases and associations with disease severity
Scientific Reports (2024)
-
Severe pediatric COVID-19: a review from the clinical and immunopathophysiological perspectives
World Journal of Pediatrics (2024)
-
Steigende Inzidenzen bei Mastoidektomien im Kindesalter
HNO (2024)