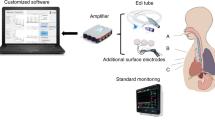
Abstract
With the development of Artificial Intelligence techniques, smart health monitoring is becoming more popular. In this study, we investigate the trend of wearable sensors being adopted and developed in neonatal cardiorespiratory monitoring. We performed a search of papers published from the year 2000 onwards. We then reviewed the advances in sensor technologies and wearable modalities for this application. Common wearable modalities included clothing (39%); chest/abdominal belts (25%); and adhesive patches (15%). Popular singular physiological information from sensors included electrocardiogram (15%), breathing (24%), oxygen saturation and photoplethysmography (13%). Many studies (46%) incorporated a combination of these signals. There has been extensive research in neonatal cardiorespiratory monitoring using both single and multi-parameter systems. Poor data quality is a common issue and further research into combining multi-sensor information to alleviate this should be investigated.
Impact statement
-
State-of-the-art review of sensor technology for wearable neonatal cardiorespiratory monitoring.
-
Review of the designs for wearable neonatal cardiorespiratory monitoring.
-
The use of multi-sensor information to improve physiological data quality has been limited in past research.
-
Several sensor technologies have been implemented and tested on adults that have yet to be explored in the newborn population.
Similar content being viewed by others
Introduction
The neonatal period is the most vulnerable time for a child’s survival, with 2.4 million deaths globally in 2020.1 The major causes of mortality are infections, birth asphyxia, and preterm birth.1,2,3 Cardiorespiratory monitoring is crucial for the early detection, prediction of prognosis, and continued monitoring of these conditions to assist clinicians in minimising morbidity and mortality.4,5
Wearable technology offers the convenience and potential of continuous cardiorespiratory monitoring in both hospital and home environments. These technologies have come in the form of adhesive patches; chest and abdominal belts; wrist and ankle bands, and clothing to measure breathing, heart activity and oxygen saturation. Many systems integrate multiple sensors for more accurate measurements and sufficient information for clinical and artificial intelligence (AI) decision-making.
The objective of this study is to review AI-driven wearable technologies for neonatal cardiorespiratory monitoring. This is achieved in a two-part review. In this Part 1: Wearable Technology, we reviewed (Sections “Review Methodology” and “Wearable Technology”), compared (Section “Discussion”) and identified gaps (Section “Future Directions”) in the current state-of-the-art in sensor types and designs of wearables being used. In Part 2: AI, machine learning techniques developed and/or suitable for analysis of cardiorespiratory monitoring are reviewed.
Review methodology
A query string was constructed to restrict to:
-
1.
Neonatal population
-
a.
Search terms: “Neonatal”, “Neonate”, “Pediatric”, “Paediatric”, “Infant”, “Baby”, “Babies”, “NICU”
-
a.
-
2.
Wearable technology
-
a.
Search terms: “Wearable”, “Textile”, “Clothing”, “Clothes”, “Garment”, “Vest”, “Jacket”, “Belt”, “Diaper”, “Sock”, “Shoe”, “Portable”, “Wireless”, “Fabric”
-
a.
-
3.
Cardiorespiratory monitoring
-
a.
Search terms: “Cardi*”, “Heart”, “Respiratory”, “Respiration”, “Lung”, “Breath”, “Breathing”, “ECG”, “Pulse”, “Oximeter”, “Apnea”, “Apnoea”, “Blood Pressure”, Vital”, “Multi*”, “SIDS”, “Sudden Infant Death Syndrome”, “SpO2”, “Oxygen”
-
a.
PubMed, Scopus, and IEEE Xplore were searched with the query string for articles published from the year 2000 onwards based on title, abstract and keywords on 09 March 2022.
The search resulted in 1,262 non-duplicate articles, which were then removed based on the following criteria:
-
Not within scope
-
Not neonatal target population
-
Not wearable devices e.g., video and radar monitoring, and sensor-integrated pillow, mattress and cot were common results excluded.
-
Not cardiorespiratory monitoring i.e., did not provide information on heart rate (HR), cardiac cycle, respiratory rate (RR), breathing volume, work of breathing, or oxygen saturation
-
-
Only abstract available
-
Insufficient information provided on wearable technology
-
Not in English or English language translation not available
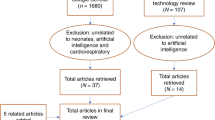
In total 88 articles including 13 review papers were obtained. The reference list of the papers, including articles citing them was searched to find additional articles. Additionally, two authors (CS and EG) independently searched for further articles, identifying an extra 32 papers. The 13 review papers were then excluded. The PRISMA flow diagram (Fig. 1) summarises the process.
PubMed (n1 = 115 papers), Scopus (n2 = 1066 papers) and IEEE Xplore (n3 = 141 papers) were searched generating a total of 1262 papers. After exclusion criteria were applied 88 papers remained. Review papers (13 papers) were removed and sensor-related articles (32 papers) were added, generating a total of 107 papers for final review.
All the selected manuscripts were then reviewed in detail by one author (EG). The articles were first categorised based on:
-
Sensors used
-
Parameters/measurements obtained from the sensors
-
The location of the sensors
-
The type of wearable modality the sensor was integrated into
-
If any machine learning algorithms were utilised
Further information extracted when available from papers included:
-
Sensor functionality and relationship to the physiological parameter
-
Cardiorespiratory conditions monitored
-
Power information
-
Relevant discussions and results on:
-
Accuracy
-
Ease-of-use
-
Robustness to noise
-
Advantages and disadvantages
-
-
Cost
The results are shown in (Table 1) and Section “Wearable Technology”.
Wearable technology
Sensor types
Sensors are transducer devices that detect a physical parameter and convert it to a signal that can be processed. For this section, sensors are classified based on the technical domain they detect and summarised in (Fig. 2).
Mechanical transducers
Strain and pressure sensors are used for respiratory health assessment, such as RR monitoring and apnoea detection. Dandekar and colleagues6,7,8 utilised a strain gauge sensor in combination with passive radio-frequency identification (RFID). The sensor was made from conductive and non-conductive threads to create an antenna, which was inductively coupled to an RFID chip. This system was integrated into an elastic band worn around the abdomen or chest of the infant. The Received Signal Strength Indicator (RSSI) from the RFID chip can then be continuously monitored by an external sensor. During inhalation, the band stretches, decoupling the antenna and RFID chip, which decreases the RSSI returned.6,7,8 Being passive and batteryless enables the system to be small and flexible.6,8 However, the RSSI is a weak signal prone to motion artefacts and temporal distortions, making signal processing and AI necessary to extract breathing/non-breathing periods.7,9,10,11
More recently, a secondary strain-based RFID tag was placed on the upper infant’s shoulder.9,10 The second RFID tag is not subject to respiratory motion and enables modelling and removal of noise artefacts present in the abdominal band’s signal.9,10 Overall, signal quality improved by >17dB,10 and breathing/non-breathing detection by 7.7% to 86%9 with the secondary sensor.
Most recently, Bellypatch was used to improve signal strength. Bellypatch is an abdominal belt that has a read range of 580 cm, nearly ten times more than the previous model.11 A larger read range improves the utility of the system and minimises radiofrequency exposure.8 The sensing property for the Bellypatch is compression. During inhalation, the torso exerts pressure on the Bellypatch, which reduces its radiation efficiency, leading to a decrease in RSSI.11
In similar research, chest or abdominal bands using strain sensors12,13 have been integrated into clothing to measure breathing. The strain sensors were made from electrically conductive strands knitted into various patterns, that change resistance when stretched.12,13 Careful selection of knit pattern was required to ensure a repeatable linear relationship between resistance and strain changes.12 Additionally, conductive strand-based sensors are prone to drift in the recorded signal, which needed to be corrected.13
Pressure sensors have been integrated into chest belts for monitoring breathing.14,15,16 These systems have high accuracy in breathing detection (99%) but require a battery system.14,16 It was found that the Velostat pressure sensor with Jersey knit exhibited the best resistance to pressure curve.16
Ballistocardiogram (BCG) is for monitoring the mechanical activity of the heart. BCG recordings are obtained using sensors that measure the forces on the surface of the body. BCG is similar to an electrocardiogram (ECG), while also being small and not requiring skin contact. BCG sensors have been integrated into clothing and used modified ECG code to determine the HR.17
Accelerometers measure acceleration along a predefined axis or a combination of axes for dual-/tri-axial accelerometers. Through the integration of the acceleration signal, velocity and displacement can also be provided. A common use of accelerometers is determining the RR when placed around the abdomen or sternum.13,18,19,20 Although this method is susceptible to motion artefact,20 two dual-axis accelerometers around the abdomen alleviates this issue.18,19 With multiple accelerometers and reference spirometer look-up table, breathing volume can also be calculated.19
Accelerometers placed on the chest can obtain seismocardiogram (SCG) and vocal biomarkers.21,22 SCG is produced from the small vibrations of the chest due to sounds and is used to obtain HR, systolic interval, pre-ejection period, and left-ventricular ejection times. It was found that HR estimation using SCG was within FDA regulation of ±5 beats per minute.21
Microphones convert sound waves to electrical signals. An omnidirectional microphone has been placed on either the neck or suprasternal notch with adhesive tape to monitor breathing.23 During breathing, the sound is created by turbulence in the respiratory system, enabling the detection of inhalation and exhalation. It was also suggested breathing volume may be determined, however, the relationship between breathing sound and breathing volume is questionable.23 A two-microphone system that was placed either on the chest, neck, or wrist to obtain a phonocardiogram (PCG) has also been proposed.24 PCG refers to heart sounds and requires no electrical contact and is low-power, making a batteryless radiofrequency powered system possible.24
Electrical transducers
ECG is for monitoring the heart’s electrical activity, through the placement of electrode sensors around the heart. With ECG, HR and cardiac abnormalities can be detected. For wearables, past work has used 2-,19,25,26,27 3-28,29,30 and 6-electrode31 configurations integrated into clothing or chest belt. Unlike traditional ECG electrodes, capacitive dry electrodes are used.29
A downside to capacitive electrodes compared to gel electrodes is the higher impedance and the need to maintain sufficient pressure for skin-to-electrode coupling/contact. These result in greater sensitivity to motion artefacts from the baby/clothes, weaker signals and disconnection.27,29,30,32,33 Additionally, when integrated into clothing which as an insulator, the detected waveforms are more distorted.29 With these limitations, typically only heartbeats are extracted, and tight-fitting clothing is required.19
To address these issues, a smart algorithm for a 6-electrode jacket was developed.31,34 The context-aware algorithm determines the optimal selection of electrodes to produce the best quality ECG signal. Therefore, the signal quality of the overall system is not affected by a single electrode disruption.34
A batteryless ECG system using RFID embedded into clothing or a chest patch has also been made.8,25 Using a heartbeat detection circuit, the RFID tag is turned off momentarily for each ECG spike detected.8,25
Commercially, NeoBeat,35 an abdominal belt has been developed. NeoBeat has two dry electrodes located on the lower back of the newborn for specifically HR monitoring.35
With two electrodes placed around the diaphragm and one reference electrode placed at the sternum, this provides monitoring of both heart and lung health.36,37,38,39,40 The primary signal obtained is a diaphragmatic electromyogram (dEMG). dEMG is a direct measure of breathing and diaphragmatic work of breathing. ECG which is present as noise can also be extracted with signal processing.36,38
Another sensor system is electrical impedance tomography (EIT). EIT utilises a belt containing multiple (1641,42 or 3243) active electrodes around the chest to monitor lung volume changes in real-time (up to 49 Hz.41) The active electrodes inject small alternative currents, which are then measured as potentials by the electrodes. The impedance of the chest is calculated from this, providing information about lung aeration and ventilation.41 Whilst real-time lung imaging is provided, these images are of low resolution, and do not represent structural lung information. Due to these issues, these lung images are difficult to be interpreted by clinicians.43 The electrodes can be used to obtain ECG.42
Magnetic transducers
A vest to measure volume changes when the neonate is breathing has been developed.44 The vest covers the entire chest and abdomen with two continuous wire coils sewn into it. Alternating current is passed through the coils and external magnetic field sensors detect this. During breathing, the volume contained within the coils changes, causing a proportional change in the magnetic field generated.44 The vest provides a direct measurement of the respiratory volume changes and does not require calibration.44
Thermal transducers
Many studies have proposed a system that can be placed under the nasal cavity or integrated with the respiratory support system to monitor breathing and apnoea.45,46,47,48,49 A polyvinylidene fluoride (PVDF) film-based sensor was utilised for this purpose. PVDF senses heat changes during breathing, that are transduced into an electric charge. PVDF is a low-power sensor, which when combined with a piezoelectric transducer for energy harvesting, a self-powered system is feasible.45,46,47,48,49
Radiant transducers
Optical-based methods are used for pulse oximetry and photoplethysmography (PPG). Pulse oximetry is the measurement of blood oxygenation saturation, that is, the relative concentration of oxygenated and de-oxygenation haemoglobin.50,51 Whereas PPG is the measurement of blood volume changes, which indicates blood perfusion to a particular area and provides heartbeat information. Both these methods utilise a combination of a light-emitting source (e.g., light-emitting diodes (LEDs)) and a light-detecting sensor (e.g., a photodiode). For pulse oximetry, oxygenated and deoxygenated haemoglobin have differing light absorption spectrums, hence, the effective attenuation of light emitted to the light detectors indicates their relative concentration. For PPG, the presence of blood absorbs light more than the absence of blood, hence, the effective attenuation of light emitted to the light detectors can indicate the volume of blood flowing through a particular area.
There has been extensive research into the usage of wearable optical sensors for HR52,53,54,55 and peripheral oxygen saturation.54,56,57 These sensors have been integrated into a sock,56 foot band52,57 and head cap55,58 for mainly home-based monitoring.53,54,56,57
Flexible silicone patches with LEDs and light sensors have been used on the neonate’s forehead.50,59,60,61 Utilising multiple near-infrared LEDs, cerebral tissue oxygenation, peripheral oxygenation and HR can be obtained. The measurement of brain oxygenation is especially important for premature infants, who are vulnerable to hypoxic and ischaemic cerebral insults that can lead to long-term morbidity. Bilirubin levels have also been measured for the early detection of neonatal jaundice.60,61
Commercially, a Bluetooth wireless cap with a forehead-mounted sensor that uses reflectance-mode optimised 525 nm light PPG to measure HR has been developed.55,58,62 By capturing blood flow at the forehead, it is less susceptible to poor peripheral perfusion.63,64 The cap is compatible with respiratory equipment, making it suitable for neonatal care.55
Other commercial devices, Baby Vida and Owlet Smart Sock, integrate the optical-based sensor into a sock to obtain oxygen saturation and HR.65,66 Owlet Smart Sock has been a popular consumer device, offering an alarm system for when oxygen saturation is too low or HR is too high, which has been perceived to reduce the anxiety of parents and prevention of critical events.65,67 However, these two monitors are not FDA-regulated.67 Additionally, it was found that Baby Vida never correctly detected hypoxemia and displayed falsely low pulse rates, whereas Owlet Smart Socket detected hypoxemia but performed inconsistently.66
The combination of fibre optics and photodiode sensors can be used for monitoring breathing.68 Optical fibres arranged in a sinusoidal pattern were integrated into an elastic material and attached to the outside of a diaper. During inhalation, the diameter of the optical fibre bend increases, which causes the intensity of the light emitted from the optical fibre to increase, which is detected by the photodiode.68 Careful design of the diameter and curvature of the optical fibres is required to ensure a sufficient linear relationship between power output and strain.68
Integrated wearable sensors
Clothing
Sensor-integrated clothing has come in the form of attachments or is fully integrated into onesies/vests/jackets, diapers, socks, and shoes. Common sensors have included inertial, optical, temperature and electrode sensors.
Chen and Bouwstra et al. have developed a smart neonatal jacket that integrates 6-electrode ECG,31,69 pulse oximeter51 and temperature sensors70 with wireless power supply and transmission.71,72,73 Similarly, baby vests to monitor respiration using resistive strain sensors around the chest and abdomen, as well as HR from a 3-/4-electrode ECG are common.32,74,75 Linti et al.’s74 vest also measured temperature from two thermistors around the armpit and humidity using electrodes that measure moisture-dependent resistance on the back. Whereas Mastro et al.’s75 vest contained optical sensors in the sleeves. For power supply, systems have been wired,74,75 wireless32 or accompanied by a plush toy to supply power.71,72,73
More recently, Chen and colleagues76,77 designed a smart vest with two textile-based dry ECG electrodes on the chest, a strain sensor around the abdomen for respiration measurement, and two inertial sensors (accelerometer, gyroscope, and magnetometer) on the wrists for movement information. The electrodes are designed to be disposable and replaceable to ensure optimal ECG signals. Additionally, the electrode is made from a cotton component sandwiched between two electronic-textile components. This sandwiched design provides a cushioning effect that improves electrode-to-skin contact during movement, improving the robustness of the ECG signal.76,77
Smart jackets have also been designed for blood pressure monitoring.78 With an expandable arm cuff within the jacket, the brachial artery can be semi-occluded and detected by the PPG sensor. Using the combination of pressure measurements from the capacitive force sensor and the PPG signal, blood pressure can be calculated.78
Baker79 designed a swaddle with two integrated sensors that each contain a temperature sensor and electrode. The sensors are placed on the posterior and anterior of the infant’s torso. The electrodes obtain ECG and hydration based on resistivity measurements between them.79
Commercially, the Goldilocks Suit and MonBaby Smart Button have been developed for home monitoring.80,81 The Goldilocks Suit is a baby onesie to monitor temperature, breathing and movement.80 Whereas MonBaby Smart Button attaches to an article of clothing to monitor breathing and movement.81
Many devices have been developed in the form of a sock or shoe.82,83,84,85,86 All devices utilised a pulse oximeter for HR and oxygen saturation. A temperature sensor was used for instantaneous peripheral temperature measurement;82,83,85,86,87 a tri-axial accelerometer was used for position, activity monitoring and to reduce motion artefacts in PPG;84,85,86 and pulse oximeter signals and galvanic skin response were obtained in a subset of the devices.87 InfaWrap82,83 and Leier and Jervan’s85,86 devices are comparatively bulky and have hard outer casings, whereas BBA bootee’s84 sensors are encapsulated in a soft fabric and is smaller.
Sensors have been designed to attach to a diaper.88,89 Mahmud et al.‘s88 attachment had two capacitive electrodes for non-contact ECG and an accelerometer. The accelerometer was used as a reference signal for an adaptive filter to remove motion artefacts and interference present in the ECG.88 NAPPA,89 a diaper cover, contained accelerometer and gyroscope sensors, for RR and body posture data.
Xu et al.90 developed a reusable system using a laser-induced graphene-based integrated flexible sensor system, that is placed within the diaper. The electrodes were used in three configurations for tilt, strain, and humidity sensing. The tilt sensor determined the rotation of the baby, the strain sensor for RR and the humidity sensor for wetness monitoring.90
Commercially for home monitoring, Snuza Hero and Levana oma sense are two diaper clip-based sensors.91,92 Both monitor abdominal movement for breathing and gently vibrate to rouse the baby if no breathing movement is detected.91,92
Patch
Roger and colleagues have developed a soft and flexible 2-patch system.21,93,94,95 One patch is placed on the hand or foot for PPG and oxygen saturation from optical-based sensors21,93 and peripheral temperature measurement.21 The other patch is placed on the back or chest and contains 2 electrodes for ECG,21,93 tri-axial accelerometer, and temperature measurement unit.21 The two patches are time synchronised for pulse transit time to be calculated from the time difference between the R-peak of the ECG chest unit and the valley regions of the PPGs on the limb unit. This pulse transit time can then be used as a surrogate measure of systolic blood pressure, which has had promising results (mean error <5 mmHg and standard deviation 8.7 mmHg).21,93,94 The tri-axial accelerometer is used for RR, posture, movement measurements, and SCG.21 In some designs, they were also batteryless.21,93 Instead, radiofrequency power transfer was supplied from an antenna placed underneath the neonate’s mattress, to the antenna on the patch.21,93
This work has translated to a start-up, Sibel Health.96 The product has been positively received for its non-invasiveness, portability, ease-of-use and ability to measure multiple vital signs in hospitals in Kenya.97,98 However, there were concerns related to cost, maintenance, lack of reliable access to electricity, and overcrowding in neonatal intensive care.97,98
De Clercq and Puers99 proposed a three-node system that was attached to the chest and abdomen. Two nodes were utilised for respiration measurement based on inertial measurements from an accelerometer and gyroscope, and one node was used for HR measurement based on ECG.99
Band
Bands suitable for placement on the ankle or wrist have been developed.100 These bands contained optical sensors for oxygen saturation and HR, and a temperature sensor.100
Belt
Belts have been placed either around the chest/thoracic wall101,102,103 or abdomen.33,104,105,106 A combination of ECG textile electrodes and inertial sensors for monitoring HR and RR respectively are common.101,103,104 Actidiaper, an elastic waistband, is placed over the abdomen.104 Actidiaper contained two textile electrodes at the back, and a commercial inertial sensor, Movesense, that clipped onto the front of the belt.107 Movesense contained a tri-axial accelerometer, gyroscope, and magnetometer, each of which was tested for RR detection, with the gyroscope y-channel producing the best results.104
In other abdominal belts, they have contained a thermistor and inertial sensors for hypothermia detection and RR respectively.102,105 Lin et al.’s102 belt contained a carbon monoxide sensor to monitor the environment. In another design, an accelerometer was wrapped around NeoBeat for breathing and ECG.33,35,106 For belts wrapped around the chest of the baby, they contained ECG electrodes for HR, an accelerometer for posture and RR, and temperature monitoring capabilities.101,103
Discussion
For determining a suitable sensor and wearable modality, power system, energy consumption, physiological information provided, accuracy, robustness to noise, ease-of-use and cost should be considered and compared (Tables 2 and 3).
For power systems, battery, battery with wireless charging, batteryless with wireless power, and passive batteryless systems have been proposed. Batteryless allows for smaller and more flexible systems not constrained by battery life. However, they are constrained by the wireless power unit or wireless reader range. Additionally, passive systems have a weaker signal, typically resulting in only HR and/or RR information being extracted.
For energy consumption, all mechanical sensors, PVDF and ECG monitoring have been shown to be capable of low-power consumption and applicable for a passive system. Whereas the magnetic field sensor and EIT system require the most energy for usage.
For cardiac monitoring, sensors have been used to provide BCG, SCG, PCG, ECG and PPG information. ECG is considered the gold standard, providing typically the highest quality signal enabling the most accurate HR estimation and extraction of information on the cardiac cycle. Similarly, PCG, SCG, BCG, and PPG can provide electrical or mechanical cardiac cycle time intervals, however, are more susceptible to noise. So far PCG, SCG, and BCG have been constrained to just heartbeat detection in the neonatal wearable space. Further research into the ability to obtain more detailed cardiac information is therefore required. Optical sensors uniquely provide both PPG and pulse oximetry, with oxygen saturation from pulse oximetry being an important vital sign not obtained by other sensor types.
For respiratory monitoring, most of the sensors only provide basic breathing monitoring for RR and apnoea detection. Multi-accelerometer, magnetic field sensor, and EIT provide more detailed information on breathing volume and work of breathing. With the magnetic field sensor being the only system directly measuring lung volume. Both accelerometers and microphones can also be used for vocal biomarkers such as adventitious lung sounds. Microphones are more appropriate to collect these lung sounds due to frequency range and accuracy, but pick up background noise. Whereas, accelerometers are not affected by background noise, though are affected by motion artefacts.
All wearable modalities have integrated multiple sensors to monitor multiple cardiorespiratory parameters. Placement of sensors around the chest is the most suitable as it maximises the types of sensors that can be used, the proximity to the heart and lungs, and related chest movements during breathing. Whereas systems placed around the abdomen or back are prone to picking up motion artefacts and EMG noise when collecting ECG.
Adhesive patches provide direct skin contact, which allows for higher quality signals due to reduced motion artefacts, closer to the source of the signal, and better electrode contact. Some adhesive patches are disposable, which increases costs and is more suited for shorter-term hospital monitoring. Adhesive patches also carry the risk of skin damage, especially for premature neonates that have fragile skin. However, many advances in adhesive technology have minimised skin damage risk.
Integration into clothing provides ease-of-use in both clinical and home environments. However, these systems need to be washable. Additionally, proper fitting is required to provide sufficient contact and reduced motion artefact for good-quality signals. This is especially a challenge as neonates vary greatly in size, and as they grow from 0 to 1 month old.
Cost is difficult to determine and compare as most of the wearables reviewed are in the research stage and costing is multifactorial. Generally, most sensors are relatively low-cost and many aim for home and/or rural usage. Disposable modalities would increase costs in the long term. Supplementary Table 1 shows the cost of existing commercial devices.
Future directions
Many newborn wearable technologies integrate multiple sensors. This provides multiple physiological signals for a clinician/machine learning algorithm to interpret, as well as fusing the information for new health parameters and improved signal quality and accuracy. This has been achieved through utilising accelerometer signals to remove motion artefacts in PPG, oxygen saturation and ECG signals,84,85,88 multi-electrode systems to improve ECG signal quality31,34 and using the combination of ECG and PPG signals to determine systolic blood pressure.21 In future, additional benefits in utilising the multiple sensors to better extract, separate and segment heart and lung signals can be achieved. For instance, ECG monitoring is contaminated with EMG signals from breathing and vice versa,36,38,108 audio signals contain both heart and lung sounds,109,110,111 and accelerometer data contain both SCG and chest movement data.21
For sensor technology, four gaps and possible future directions are presented. Firstly, existing pressure-based sensors can also be used for heart monitoring via apexcardiogram.112 Apexcardiogram provides information on the changing volume of the left ventricle and cardiac function information.112 Secondly, microphone sensors have been used for either heart or lung information.23,24 With more suitable housing of the microphone within a diaphragm structure, like stethoscopes, both heart and lung sounds can be obtained simultaneously and more accurately.113 Thirdly, there is research into flexible and wearable ultrasound transducers.114 Ultrasound provides echocardiography, which is common for detailed information on newborn cardiac function such as blood pressure and cardiac output.114,115 Finally, there are existing chemical sensors for expiratory gas content.116 Whilst a wearable version of these sensors does not exist, a badge-type clothing attachment has been proposed.
This study has focused on wearable cardiorespiratory monitoring. In future, a review of non-contact cardiorespiratory methods such as video and radar monitoring, and sensors integrated into a pillow, mattress and cot that offer similar benefits to wearables should be completed.
Conclusions
We reviewed wearable technologies for neonatal cardiorespiratory monitoring. There has been extensive research in both single-parameter and multi-parameter sensing. Multi-sensor systems offer the possibility of improved accuracy in the acquisition of physiological signals and subsequent training accuracy due to the variety of heart and lung information provided to machine learning models.
In Part 2: AI, the developments of the machine learning techniques used for these wearable neonatal cardiorespiratory monitoring devices are explored. Additionally, machine learning techniques suitable for neonatal cardiorespiratory monitoring are examined.
References
UNICEF. Neonatal mortality. Accessed 12/01/2020, 2020. https://data.unicef.org/topic/child-survival/neonatal-mortality/
World Health Organisation. Newborn Death and Illness. Accessed 12/01/2020, 2020. https://www.who.int/pmnch/media/press_materials/fs/fs_newborndealth_illness/en/
World Health Organisation. Newborns: improving survival and well-being. Accessed 12/01/2020, 2020. https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality
Gleason C. A., Juul S. E. Avery’s diseases of the newborn e-book. Elsevier Health Sciences; 2017.
World Health Organisation. Preterm Birth. Accessed 03/23/2022, 2022. https://www.who.int/news-room/fact-sheets/detail/preterm-birth
Patron, D. et al. On the use of knitted antennas and inductively coupled RFID tags for wearable applications. IEEE Trans. Biomed. circuits Syst. 10, 1047–1057 (2016).
Mongan W. et al. A multi-disciplinary framework for continuous biomedical monitoring using low-power passive RFID-based wireless wearable sensors. IEEE; 2016:1-6.
Vora S. A. et al. On implementing an unconventional infant vital signs monitor with passive RFID tags. 2017 IEEE International Conference on RFID (RFID) 47–53 (2017).
Acharya, S. et al. Ensemble learning approach via kalman filtering for a passive wearable respiratory monitor. IEEE J. Biomed. health Inform. 23, 1022–1031 (2018).
Hansen S. et al. Fusion learning on multiple-tag RFID measurements for respiratory rate monitoring. 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE); 472–480 2020.
Tajin, M. A. S., Amanatides, C. E., Dion, G. & Dandekar, K. R. Passive UHF RFID-based knitted wearable compression sensor. IEEE internet things J. 8, 13763–13773 (2021).
Jakubas, A. & Łada-Tondyra, E. A study on application of the ribbing stitch as sensor of respiratory rhythm in smart clothing designed for infants. J. Text. Inst. 109, 1208–1216 (2018).
Munz, M. & Wolf, N. Simulation of breathing patterns and classification of sensor data for the early detection of impending sudden infant death. Curr. Directions Biomed. Eng. 5, 401–403 (2019).
Cay G. et al. Baby-Guard: An IoT-based neonatal monitoring system integrated with smart textiles. 2021 IEEE International Conference on Smart Computing (SMARTCOMP); 129–136 (2021).
Altekreeti A. et al. NAPNEA: A cost effective neonatal apnea detection system. IEEE; 2021:113-114.
Cay G. et al. An E-textile respiration sensing system for NICU monitoring: design and validation. J. Signal Process. Syst. 94, 543–557 (2021).
Raknim P., Lan K.-C., Linker Y.-C., Lu Y.-T. Position: On the Use of Low-cost Sensors for Non-intrustive Newborn Sepsis Monitoring. In The 5th ACM Workshop on Wearable Systems and Applications. 2019:39-40.
Clercq H. D., Jourand P., Puers R. Textile integrated monitoring system for breathing rhythm of infants. Springer; 2010:525-528.
Jourand, P., De Clercq, H. & Puers, R. Robust monitoring of vital signs integrated in textile. Sens. Actuators A: Phys. 161, 288–296 (2010).
Raj A. A, Preejith S., Raja V. S., Joseph J., Sivaprakasam M. Clinical validation of a wearable respiratory rate device for neonatal monitoring. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 1628–1631 (2018).
Chung, H. U. et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 26, 418–429 (2020).
Jeong H. et al. Miniaturized wireless, skin-integrated sensor networks for quantifying full-body movement behaviors and vital signs in infants. Pro. Natl. Acad. Sci. 118, 1–10; (2021).
Corbishley, P. & Rodriguez-Villegas, E. Breathing detection: towards a miniaturized, wearable, battery-operated monitoring system. IEEE Trans. Biomed. Eng. 55, 196–204 (2007).
Mandal, S., Turicchia, L. & Sarpeshkar, R. A low-power, battery-free tag for body sensor networks. IEEE Pervasive Comput. 9, 71–77 (2009).
Agezo S. et al. Battery-free RFID heart rate monitoring system. IEEE; 2016:1-7.
Lavizzari, A. et al. Heart‐rate agreement between ECG and a new, wireless device during early skin‐to‐skin contact. Acta Paediatrica 110, 1803–1809 (2021).
Nikolova E., Ganev B., Gieva E. Wearable intelligent textile suits for telemetry monitoring in pediatrics. 2021 XXX International Scientific Conference Electronics (ET); 1–6 (2021).
Younessi Herav, M. A. Design and construction of wearable electrocardiogramto monitor cardiac activity in infants. J. North Khorasan Univ. Med. Sci. 5, 1031–1036 (2014).
Ueno A. A system for detecting electrocardiographic potential through underwear worn by an infant from its dorsal surface. In Proc. World Congress on Medical Physics and Biomed. Eng. Vol. 14, 497-500 (2006).
Coosemans, J., Hermans, B. & Puers, R. Integrating wireless ECG monitoring in textiles. Sens. Actuators A: Phys. 130, 48–53 (2006).
Chen W. et al. Design of wireless sensor system for neonatal monitoring. In 2011 4th IFIP International Conference on New Technologies, Mobility and Security. 1–5 (IEEE, 2011).
Catrysse, M. et al. Towards the integration of textile sensors in a wireless monitoring suit. Sens. Actuators A: Phys. 114, 302–311 (2004).
Urdal, J. et al. Automatic identification of stimulation activities during newborn resuscitation using ECG and accelerometer signals. Comput. Methods Prog. Biomed. 193, 105445 (2020).
Bouwstra S., Chen W., Oetomo S. B., Feijs L. M., Cluitmans P. J. Designing for reliable textile neonatal ECG monitoring using multi-sensor recordings. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2488–2491 (2011).
Laerdal Global Health. Neobeat—Newborn Heart Rate Meter | Laerdal Global Health. Accessed 03/02/2022, 2022. https://shop.laerdalglobalhealth.com/product/neobeat/
Van Leuteren, R. W. et al. Cardiorespiratory monitoring in the delivery room using transcutaneous electromyography. Arch. Dis. Child.-Fetal Neonatal Ed. 106, 352–356 (2021).
van Leuteren, R. W., de Waal, C. G., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. Transcutaneous monitoring of diaphragm activity as a measure of work of breathing in preterm infants. Pediatr. Pulmonol. 56, 1593–1600 (2021).
van Leuteren, R. W. et al. Diaphragmatic electromyography in preterm infants: the influence of electrode positioning. Pediatr. Pulmonol. 55, 354–359 (2020).
Kraaijenga, J. V. et al. Classifying apnea of prematurity by transcutaneous electromyography of the diaphragm. Neonatology 113, 140–145 (2018).
Kraaijenga, J. V., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. Transcutaneous electromyography of the diaphragm: A cardio‐respiratory monitor for preterm infants. Pediatr. Pulmonol. 50, 889–895 (2015).
Wu Y., Langlois P., Bayford R., Demosthenous A. Design of a CMOS active electrode IC for wearable electrical impedance tomography systems. 2016 IEEE International Symposium on Circuits and Systems (ISCAS); 846–849 (2016).
Wu, Y., Jiang, D., Bardill, A., Bayford, R. & Demosthenous, A. A 122 fps, 1 MHz bandwidth multi-frequency wearable EIT belt featuring novel active electrode architecture for neonatal thorax vital sign monitoring. IEEE Trans. Biomed. Circuits Syst. 13, 927–937 (2019).
Vahabi, N. et al. Deep analysis of EIT dataset to classify apnea and non-apnea cases in neonatal patients. IEEE Access 9, 25131–25139 (2021).
Olden, C., Symes, E. & Seddon, P. Measuring tidal breathing parameters using a volumetric vest in neonates with and without lung disease. Pediatr. Pulmonol. 45, 1070–1075 (2010).
Mahbub I. et al. A low power wireless apnea detection system based on pyroelectric sensor. 2015 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS); 1–3 (2015).
Mahbub I. et al. Design of a pyroelectric charge amplifier and a piezoelectric energy harvester for a novel non-invasive wearable and self-powered respiratory monitoring system. 2017 IEEE Region 10 Humanitarian Technology Conference (R10-HTC); 105–108 (2017).
Mahbub I. et al. A low power wearable respiration monitoring sensor using pyroelectric transducer. 017 United States National Committee of URSI National Radio Science Meeting (USNC-URSI NRSM); 1–2 (2017).
Shamsir S. et al. Instrumentation of a pyroelectric transducer based respiration monitoring system with wireless telemetry. 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC); 1–6 (2018).
Shamsir S., Hassan O., Islam S. K. Smart infant-monitoring system with machine learning model to detect physiological activities and ambient conditions. 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC); 1–6 (2020).
Kleiser, S. et al. In vivo precision assessment of a near-infrared spectroscopy-based tissue oximeter (OxyPrem v1. 3) in neonates considering systemic hemodynamic fluctuations. J. Biomed. Opt. 23, 067003 (2018).
Chen W., Ayoola I., Oetomo S. B., Feijs L. Non-invasive blood oxygen saturation monitoring for neonates using reflectance pulse oximeter. 2010 Design, Automation & Test in Europe Conference & Exhibition (DATE 2010); 1530–1535 (2010).
Dave A. J. Wearable Wireless Sensors for Neonatal Health Monitoring in the NICU. California State University, Northridge; 2018.
Dhumal, S., Kumbhar, N., Tak, A. & Shaikh, S. Wearable health monitoring system for babies. Int. J. Computer Eng. Technol. (IJCET) 7, 15–23 (2016).
Ruiz A., Córdova P., Gordón C. Telemedicine system to avoid sudden death syndrome by continuous monitoring of vital signs. 2018 International Conference on eDemocracy & eGovernment (ICEDEG); 212–217 (2018).
Henry, C. et al. Accurate neonatal heart rate monitoring using a new wireless, cap mounted device. Acta Paediatrica 110, 72–78 (2021).
Datcu M., Luca C., Corciova C. Smart wearable SpO 2 monitor for newborns. Springer; 2019:41-44.
Harris, B. U. et al. Accuracy of a portable pulse oximeter in monitoring hypoxemic infants with cyanotic heart disease. Cardiol. Young-. 29, 1025–1029 (2019).
Surepulse. Products - Surepulse. Accessed 03/17/2022, 2022. https://www.surepulsemedical.com/products/
Rwei, A. Y. et al. A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc. Natl Acad. Sci. 117, 31674–31684 (2020).
Inamori G. et al. Wearable multi vital monitor for newborns. 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS); 337–339 (2020).
Inamori, G. et al. Neonatal wearable device for colorimetry-based real-time detection of jaundice with simultaneous sensing of vitals. Sci. Adv. 7, eabe3793 (2021).
Grubb, M. et al. Forehead reflectance photoplethysmography to monitor heart rate: preliminary results from neonatal patients. Physiological Meas. 35, 881 (2014).
Fernandez, M. et al. Evaluation of a new pulse oximeter sensor. Am. J. Crit. Care 16, 146–152 (2007).
Berkenbosch, J. W. & Tobias, J. D. Comparison of a new forehead reflectance pulse oximeter sensor with a conventional digit sensor in pediatric patients. Respiratory care 51, 726–731 (2006).
Owlet. Smart Sock Baby Monitor – Owlet Australia. Accessed 03/02/2022, 2022. https://owletcare.com.au/products/owlet-smart-sock
Bonafide, C. P. et al. Accuracy of pulse oximetry-based home baby monitors. JAMA 320, 717–719 (2018).
Stiefel A. At-home cardiorespiratory monitors for newborns: helping or hurting parents’ peace of mind. Pediatr. Nursing. 47, 11–16 (2021).
Hariyanti, D. K., Aisyah F. N., Nadia K. V. A. W., Purnamaningsih R. W. Design of a wearable fiber optic respiration sensor for application in NICU incubators. AIP Publishing LLC; 2019:020002.
Bouwstra S., Chen W., Feijs L., Oetomo S. B. Smart jacket design for neonatal monitoring with wearable sensors. 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks; 162–167 (2009).
Chen, W. et al. Design of an integrated sensor platform for vital sign monitoring of newborn infants at neonatal intensive care units. J. Healthc. Eng. 1, 535–554 (2010).
Chen W., Sonntag C., Boesten F., Oetomo S. B., Feijs L. A power supply design of body sensor networks for health monitoring of neonates. 2008 International Conference on Intelligent Sensors, Sensor Networks and Information Processing; 255–260 (2008).
Chen W., Bouwstra S., Oetomo S. B., Feijs L. Intelligent design for neonatal monitoring with wearable sensors. Intell. Biosens, 386–410 (InTech, 2010).
Chen W., Bouwstra S. Smart jacket design for improving comfort of neonatal monitoring. Neonatal monitoring technologies: design for integrated solutions. IGI Global; 2012:361-385.
Linti, C., Horter, H., Osterreicher, P. & Planck, H. Sensory baby vest for the monitoring of infants. IEEE 3, 137 (2006). pp.
Mastro M., Bunalski M., BuSha B. Non-invasive neonatal vital acquisition unit. 2012 38th Annual Northeast Bioengineering Conference (NEBEC); 245–246 (2012).
Chen H. et al. A wearable sensor system for neonatal seizure monitoring. 2017 IEEE 14th International Conference on Wearable and Implantable Body Sensor Networks (BSN); 27–30 (2017).
Chen, H. et al. Design of an integrated wearable multi-sensor platform based on flexible materials for neonatal monitoring. IEEE Access 8, 23732–23747 (2020).
Monràs Álvarez D. A Novel smart jacket for blood pressure measurement based on shape memory alloys. Universitat Politècnica de Catalunya; 2019.
Baker C. R. et al. Wireless sensor networks for home health care. 21st International Conference on Advanced Information Networking and Applications Workshops (AINAW'07); 832–837 (2007).
Goldilocks. The unique Goldilocks Suit baby monitoring system. Accessed 03/04/2022, 2022. https://www.goldilockssuit.com/
MonBaby. MonBaby Smart Button: Track Your Baby’s Sleep Position and Rollover Movement During Sleep. Low Energy Bluetooth Connectivity. Accessed 03/04/2022, 2022. howpublished = https://monbabysleep.com/products/monbaby-essential
Rahim M. H. A, Adib M. A. H. M., Baharom M. Z, Hasni N. H. M. Improving the Infant-Wrap (InfaWrap) Device for Neonates Using MyI-Wrap Mobile Application. Intelligent Manufacturing and Mechatronics. Springer; 2021:239-248.
Rahim M. H. A., Adib M. A. H. M., Baharom M. Z, Sahat I. M., Hasni N. H. M. Non-invasive study: monitoring the heart rate and SpO2 of the new born using infaWrap device. 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES); 212–217 (2021).
Rimet Y. et al. Surveillance of infants at risk of apparent life threatening events (ALTE) with the BBA bootee: a wearable multiparameter monitor. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 4997–5000 (2007).
Leier M., Jervan G. Sleep apnea pre-screening on neonates and children with shoe integrated sensors. 2013 NORCHIP; 1–4 (2013).
Leier M., Jervan G. Miniaturized wireless monitor for long-term monitoring of newborns. 2014 14th Biennial Baltic Electronic Conference (BEC); 193–196 (2014).
Fletcher, R. R. et al. iCalm: Wearable sensor and network architecture for wirelessly communicating and logging autonomic activity. IEEE Trans. Inf. Technol. Biomed. 14, 215–223 (2010).
Mahmud M. S., Wang H., Fang H. Design of a wireless non-contact wearable system for infants using adaptive filter. In Proceedings of the 10th EAI International Conference on Mobile Multimedia Communications, Chongqing, China. Vol.13, 83–88 (2017).
Ranta, J. et al. An openly available wearable, a diaper cover, monitors infant’s respiration and position during rest and sleep. Acta Paediatrica 110, 2766–2771 (2021).
Xu, K. et al. A wearable body condition sensor system with wireless feedback alarm functions. Adv. Mater. 33, 2008701 (2021).
Snuza. Hero SE | Snuza Baby Breathing Monitors. Accessed 03/04/2022, 2022. https://www.snuza.com/product/hero-se/
Levana. Wearable Abdominal Movement Baby Monitor—Levana. Accessed 03/04/2022, 2022. https://www.mylevana.com/products/oma-sense
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Liu, C. et al. Wireless, skin‐interfaced devices for pediatric critical care: application to continuous, noninvasive blood pressure monitoring. Adv. Healthc. Mater. 10, 2100383 (2021).
Xu, S. et al. Wireless skin sensors for physiological monitoring of infants in low-income and middle-income countries. Lancet Digital Health 3, e266–e273 (2021).
Sibel Health. Sibel Health. Accessed 03/03/2022, 2022. https://www.sibelhealth.com/
Ginsburg, A. S. et al. Multiparameter Continuous Physiological Monitoring Technologies in Neonates Among Health Care Providers and Caregivers at a Private Tertiary Hospital in Nairobi, Kenya: Feasibility, Usability, and Acceptability Study. J. Med. Internet Res. 23, e29755 (2021).
Kinshella, M.-L. W. et al. Qualitative study exploring the feasibility, usability and acceptability of neonatal continuous monitoring technologies at a public tertiary hospital in Nairobi, Kenya. BMJ open 12, e053486 (2022).
De Clercq, H. & Puers, R. A neonatal body sensor network for long-term vital signs acquisition. Procedia Eng. 47, 981–984 (2012).
Sriraam N., Gupta S., Tejaswini S., Pradeep G. Wrist Based Wireless Vital Monitoring System for Continuous Assessment of Pre-term Neonates in NICU Environment. In 2021 3rd International Conference on Electrical, Control and Instrumentation Engineering (ICECIE).1–4 (IEEE, 2021).
Piccini, L., Ciani, O., Grönvall, E., Marti, P., & Andreoni, G. New monitoring approach for neonatal intensive care unit. In 5th International Workshop on Wearable Micro and Nanosystems for Personalized Health. 2008:6.
Lin W., Zhang R., Brittelli J., Lehmann C. Wireless Infant Monitoring Device for the prevention of sudden infant death syndrome. In 2014 11th International Conference & Expo on Emerging Technologies for a Smarter World (CEWIT). 1–4 (2014, IEEE).
Ferreira A. G. et al. A smart wearable system for sudden infant death syndrome monitoring. In 2016 IEEE International Conference on Industrial Technology (ICIT). 1920–1925 (2016, IEEE).
Acosta Leinonen J. N. Monitoring Newborn and Infant Sleep Respiration and Heart Rate with a Wearable Sensor. 2019;
Rao H. et al. Design of a wearable remote neonatal health monitoring device. Springer; 2014:34-51.
Vu, H. et al. Automatic classification of resuscitation activities on birth-asphyxiated newborns using acceleration and ECG signals. Biomed. Signal Process. Control 36, 20–26 (2017).
Movesense. Movesense – Open Wearable Tech Platform. Accessed 03/02/2022, 2022. https://www.movesense.com/
Liu Q., Poon C., Zhang Y. Wearable technologies for neonatal monitoring. Neonatal Monitoring Technologies: Design for Integrated Solutions. IGI Global; 2012:12-40.
Grooby E. et al. Neonatal heart and lung sound quality assessment for robust heart and breathing rate estimation for telehealth applications. IEEE Journal of Biomedical and Health Informatics. 2020;
Grooby E. et al. A new non-negative matrix co-factorisation approach for noisy neonatal chest sound separation. IEEE; 2021:5668-5673.
Grooby, E. et al. Real-time multi-level neonatal heart and lung sound quality assessment for telehealth applications. IEEE Access 10, 10934–10948 (2022).
Lin, J. et al. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep. Phys. Sci. 2, 100541 (2021).
Yilmaz, G. et al. A wearable stethoscope for long-term ambulatory respiratory health monitoring. Sensors 20, 5124 (2020).
La T. G., Le L. H.. Flexible and wearable ultrasound device for medical applications: a review on materials, structural designs, and current challenges. Adv. Mater. Technol. 7, 2100798 (2021).
de Boode, W.-P. Advanced hemodynamic monitoring in the neonatal intensive care unit. Clin. Perinatol. 47, 423–434 (2020).
Chan, M., Estève, D., Fourniols, J.-Y., Escriba, C. & Campo, E. Smart wearable systems: Current status and future challenges. Artif. Intell. Med. 56, 137–156 (2012).
Ostojic D. et al. Reducing false alarm rates in neonatal intensive care: a new machine learning approach. Oxygen Transp. Tissue XLI. Springer; 2020:285-290.
Daly J., Monasterio V., Clifford G. D. A neonatal apnoea monitor for resource-constrained environments. IEEE; 2012:321-324.
Mongan W. M. et al. Real-time detection of apnea via signal processing of time-series properties of RFID-based smart garments. 2016 IEEE Signal Processing in Medicine and Biology Symposium (SPMB); 1–6 (2016).
Kwak, S. S. et al. Skin‐integrated devices with soft, holey architectures for wireless physiological monitoring, with applications in the neonatal intensive care unit. Adv. Mater. 33, 2103974 (2021).
Chen W., Nguyen S. T., Coops R., Oetomo S. B., Feijs L. Wireless transmission design for health monitoring at neonatal intensive care units. 2009 2nd International Symposium on Applied Sciences in Biomedical and Communication Technologies; 1–6 (2009).
Funding
E. G. acknowledges the support of the MIME-Monash Partners-CSIRO sponsored PhD research support program and Research Training Program (RTP). T.C. K. and D. S. are supported by the National Institute of Health Research (NIHR) Children and Young People MedTech Co-operative (CYP MedTech). D. Sharkey has received funding for technology development from the Medical Research Council, NIHR and Action Medical Research, and is a non-executive director of SurePulse Medical who are developing monitoring solutions for neonatal care. A. Malhotra’s research is supported by the NHMRC (Aus) and Cerebral Palsy Alliance (Aus). The study is supported by the Monash Institute of Medical Engineering (MIME). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or of the Department of Health.
Author information
Authors and Affiliations
Contributions
Please list which authors completed each of the following criteria. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data- E. G., C. S., T. C. K., D. S., F. M., A. M.. Drafting the article or revising it critically for important intellectual content- E. G., C. S., T. C. K. D. S., F. M., A. M.. Final approval of the version to be published- E. G., C. S., T. C. K., D. S., F. M., A. M..
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grooby, E., Sitaula, C., Chang Kwok, T. et al. Artificial intelligence-driven wearable technologies for neonatal cardiorespiratory monitoring: Part 1 wearable technology. Pediatr Res 93, 413–425 (2023). https://doi.org/10.1038/s41390-022-02416-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02416-x