Abstract
Background
Postnatal lean mass accretion is commonly reduced in preterm infants. This study investigated mechanisms involved in the blunted feeding-induced activation of Akt in the skeletal muscle of preterm pigs that contributes to lower protein synthesis rates.
Methods
On day 3 following cesarean section, preterm and term piglets were fasted or fed an enteral meal. Activation of Akt signaling pathways in skeletal muscle was determined.
Results
Akt1 and Akt2, but not Akt3, phosphorylation were lower in the skeletal muscle of preterm than in term pigs (P < 0.05). Activation of Akt-positive regulators, PDK1 and mTORC2, but not FAK, were lower in preterm than in term (P < 0.05). The formation of Akt complexes with GAPDH and Hsp90 and the abundance of Ubl4A were lower in preterm than in term (P < 0.05). The abundance of Akt inhibitors, PHLPP and SHIP2, but not PTEN and IP6K1, were higher in preterm than in term pigs (P < 0.05). PP2A activation was inhibited by feeding in term but not in preterm pigs (P < 0.05).
Conclusions
Our results suggest that preterm birth impairs regulatory components involved in Akt activation, thereby limiting the anabolic response to feeding. This anabolic resistance likely contributes to the reduced lean accretion following preterm birth.
Impact
-
The Akt-mTORC1 pathway plays an important role in the regulation of skeletal muscle protein synthesis in neonates.
-
This is the first evidence to demonstrate that, following preterm birth, the postprandial activation of positive regulators of Akt in the skeletal muscle is reduced, whereas the activation of negative regulators of Akt is enhanced.
-
This anabolic resistance of Akt signaling in response to feeding likely contributes to the reduced accretion of lean mass in premature infants.
-
These results may provide potential novel molecular targets for intervention to enhance lean growth in preterm neonates.
Similar content being viewed by others
Introduction
Approximately 1 in 10 infants in the United States are born premature1 and these infants frequently exhibit extrauterine growth restriction.2 Premature infants are at increased risk of adverse health outcomes in adulthood, including obesity, diabetes, and cardiovascular disease.3,4,5 Preterm infants reaching term equivalent age have a lower whole body and regional lean body mass compared to their term-born infant counterparts.6 Mitigation efforts to promote lean growth of preterm infants through better nutritional management should have a positive impact on their long-term health.
Studies show that during early life, skeletal muscle, a major component of lean body mass, grows faster than any other tissue.7 During the neonatal period, the rapid growth of skeletal muscle is achieved mainly due to enhanced sensitivity and responsiveness of translation initiation signaling components to the postprandial rise in insulin and amino acids.8,9 Recently, using neonatal piglets, a highly translatable animal model of human infants, we demonstrated that preterm blunts the feeding-induced activation of insulin and amino acid signaling components that regulate mTORC1 activation and muscle protein synthesis.10 We showed that the postprandial activation of Akt and its downstream effectors, including mechanistic target of rapamycin complex 1 (mTORC1), p70 ribosomal protein S6 kinase 1 (S6K1), eukaryotic translation initiation factor (eIF)4E binding protein 1 (4EBP1), and the active eIF4E·eIF4G complex, was lower in preterm than in term piglets (Fig. 1).10 Moreover, using hyperinsulinemic-euaminoacidemic-euglycemic clamp studies, we demonstrated that S6K1 and 4EBP1 phosphorylation (mTORC1 signaling) and protein synthesis in skeletal muscle is attenuated in pigs born preterm compared to term in response to equivalent increases in insulin11 and suggests that the reduced translation initiation signaling in the skeletal muscle can be due, at least in part, to attenuated Akt signaling. In contrast, the activation of the insulin signaling pathway upstream of Akt, including phosphorylation of the insulin receptor (IR) and insulin receptor substrate 1 (IRS1), and IRS1–phosphatidylinositol-3-kinase (PI3K) complex abundance, were not affected by preterm birth,10 suggesting that additional unknown regulatory factors that mediate Akt activation are influenced by preterm birth. In support of this finding, a study in baboons showed that the insulin-induced phosphorylation of Akt was blunted by prematurity, while the phosphorylation of IR and IRS1 was unchanged.12
The activation of Akt is highly complex and is tightly controlled via a multistep process that depends on physiological conditions, the cell type, and types of stimuli. 4EBP1 eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1, Akt1/2/3 Akt/PKB protein kinase B 1/2/3, eEF2 elongation factor 2, eIF2α eukaryotic translation initiation factor 2α, eIF4E eukaryotic translation initiation factor 4E, eIF4G eukaryotic translation initiation factor 4G, FAK focal adhesion kinase, IP6K1 inositol hexakisphosphate kinase 1, IP7 5-diphosphoinositolpentakisphosphate, IR insulin receptor, IRS1 insulin receptor substrate 1, mTORC1/2 mechanistic target of rapamycin complex 1/2, PDK1 3-phosphoinositide-dependent protein kinase-1, PI3K phosphatidylinositol-3-kinase, PHLPP PH domain leucine-rich repeat protein phosphatase, PP2A protein phosphatase 2A, PTEN phosphatase and tensin homolog deleted on chromosome ten, rpS6 ribosomal protein S6, S6K1 p70 ribosomal protein S6 kinase 1, SHIP2 SH2 domain-containing inositol phosphatase, TSC1/2, Ulb4A, ubiquitin-like protein 4A.
Genetic studies indicate that the Akt kinase family, which consists of three homologous isoforms, Akt1, Akt2, and Akt3, functions as regulators of cellular growth, glucose homeostasis, and neural development (Fig. 1).13 While multiple factors can influence the activation of Akt isoforms, upstream signaling mechanisms exist that positively and negatively regulate Akt activation. The activation of Akt by insulin entails phosphorylation of threonine 308 and serine 473 residues, which are facilitated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and mechanistic target of rapamycin complex 2 (mTORC2), respectively.14,15,16 Focal adhesion kinase (FAK), via PI3K, also serves as a positive regulator of Akt.17,18 In addition, insulin promotes ubiquitin-like protein 4A (Ubl4A) membrane translocation, a crucial step for Akt activation.19 Negative regulation of Akt by phosphatases is also crucial for normal cellular physiology.20 Akt phosphorylation is reduced in part by the action of major phosphatases that either dephosphorylate Akt directly or degrade the lipid secondary messengers phosphatidylinositol-3,4,5-triphosphate (PIP3) that modulates PDK1 activity.20,21,22,23,24 These phosphatases include phosphatase and tensin homolog deleted on chromosome ten (PTEN), SH2 domain-containing inositol phosphatase (SHIP2), protein phosphatase 2A (PP2A), PH domain leucine-rich repeat protein phosphatase (PHLPP), and inositol hexakisphosphate kinase 1 (IP6K1), the latter of which generates 5-diphosphoinositolpentakisphosphate (IP7), a potent inhibitor of Akt.21,22,23,24 Under favorable conditions for Akt activation, the binding of Akt with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)25 or heat shock protein 90 (Hsp90)26 protects Akt from the action of phosphatases. Conversely, uncontrolled regulation by these phosphatases results in the hyper-activation of Akt.27
The blunted activation of Akt, yet unaltered insulin receptor activation, in the skeletal muscle of pigs born prematurely guided us to speculate that there are undetermined regulatory factors of Akt in skeletal muscle that are also modified by preterm birth.10 Therefore, the objective of this study was to investigate and define the molecular mechanisms underlying the differential effects of preterm birth on the feeding-induced activation of signaling components that positively or negatively regulate Akt activation in the skeletal muscle of a preterm piglet model.
Methods
Animals and surgeries
The experimental protocol was approved by the Institutional Animal Care and Use Committee, Baylor College of Medicine, and was conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals as described previously.10 Pregnant sows (Yorkshire × Landrace × Duroc × Hampshire) were obtained from a commercial farm (Burton, TX) and housed at the USDA/ARS Children’s Nutrition Research Center (Houston, TX) with ad libitum access to food and water. Piglets were obtained via cesarean delivery as previously described.10 Term piglets were delivered at 112 days to circumvent natural delivery, which occurs at or around 114 days of gestation, and preterm pigs were delivered at 103 days gestation, which corresponds to a gestational age between 30 and 32 weeks in humans.28 Pigs born at term were also delivered by C-section, so any effect of this method of delivery is applied equally to both groups. Approximately 6 h after delivery, all viable piglets were implanted with jugular vein and carotid artery catheters using sterile techniques. After surgery, piglets were placed in individual plexiglass incubators and monitored every hour with a full clinical assessment every 12 h, as described previously.10
Nutritional support and study design
As previously described,10 total parenteral nutrition (TPN), consisting of a complete mixture of amino acids, dextrose, lipid, electrolytes, vitamins, and minerals to meet or exceed the nutritional requirements of neonatal pigs, was administered via the jugular catheter at 6 mL/kg/h and was gradually increased over 48 h to 10 mL/kg/h. Piglet body weights were obtained on postnatal days 0 (at birth), 2, and 3; TPN infusion rates were calculated accordingly. By day 2, all piglets received full amounts of nutrition. On day 3, all piglets were fasted for 4 h. Randomization was used to assign piglets by birth weight to fast one additional h or to receive a complete elemental meal by oral gavage, to yield four treatment groups: Preterm-fasted (PF, n = 13 male and 10 female pigs); Preterm-postprandial (PP, n = 14 male and 11 female pigs); Term-fasted (TF, n = 10 male and 11 female pigs); Term-postprandial (TP, n = 16 male and 5 female pigs). The macronutrient composition (protein, fat, and lactose) of the enteral meal mimicked sow’s milk (40 mL fluid, 31.5 kcal energy, 1.3 g carbohydrate, 2.7 g amino acids, and 1.6 g lipid) and provided one-sixth of the daily nutrient requirements, including electrolytes, vitamins, and trace minerals.10
Protein extraction and immunoprecipitation
Immediately following euthanasia at 60 min after the elemental meal or after the additional 1-h fast, longissimus dorsi (LD) muscles were immediately frozen in liquid nitrogen and then stored at −80 °C. The extraction of proteins for pan-Akt immunoprecipitation (Akt-GAPDH and Akt-Hsp90 complex analysis) and Akt isoform immunoprecipitation (Akt1, Akt2, and Akt3 phosphorylation) or western blot analysis were carried out as previously described.9 Briefly, muscles were homogenized in 7 volumes of HEPES buffer (20 mmol HEPES, pH 7.4; 2 mmol EGTA; 50 mmol NaF; 100 mmol KCl; 0.2 mmol EDTA; 50 mmol β‐glycerophosphate) containing protease and phosphatase inhibitors (1 mmol DTT, 1 mmol benzamidine, 1 mmol Na3VO4, and a mixture of protease inhibitor cocktail/#P8340 Sigma-Aldrich, St. Louis, MO). The homogenates were cleared by centrifugation at 10,000 × g for 10 min at 4 °C. For Akt precipitation, supernatants (500 µg of protein) were incubated overnight at 4 °C with constant rocking with 2 µg anti-pan-Akt, Akt1, Akt2, and Akt 3, respectively (1:100; Rabbit mAb #4685, Rabbit mAb #75692, Rabbit mAb #3063, and Mouse mAb #8018; Cell Signaling Technology, Danvers, MA). Immunoprecipitates were recovered with goat anti-rabbit IgG and goat anti-mouse IgG magnetic beads (# 310207 and # 310007; Qiagen, Valencia, CA), washed, and resuspended in sample buffer as described elsewhere.9 For western blot analysis, the supernatants were added in an equal amount of 2× SDS sample buffer and boiled for 5 min.
For immunoprecipitation of the mTORC2 complex (mTORC2 phosphorylation), protein extraction was performed as previously described.29 CHAPS buffer was used to optimize the recovery of an intact mTORC2 complex.16 Briefly, muscle samples were homogenized in 7 volumes of CHAPS buffer (40 mM HEPES, pH 7.5, 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM β-glycerolphosphate, 40 mM NaF, 1.5 mM sodium vanadate, 0.3% CHAPS, 0.1 mM PMSF, 1 mM benzamidine, and 1 mM DTT). The homogenate was mixed and incubated on a platform rocker for 30 min at 4 °C followed by centrifugation at 1000 × g for 3 min (4 °C). The supernatant (500 µg of protein) was mixed with 2 µg of anti-Rictor antibody (1:100; #A300-459A; Bethyl Laboratories, Montgomery, TX) and incubated on a platform rocker overnight at 4 °C. The next day, the mTORC2 complex was isolated by combining the immune complexes with a goat anti-rabbit BioMag IgG (# 310207; Qiagen) bead slurry followed by incubation at 4 °C for 1 h. The bead-immune complexes were then collected in a magnetic rack by washing twice with CHAPS buffer and once in CHAPS buffer as above, except it contained 200 mM instead of 120 mM NaCl and 60 mM instead of 40 mM HEPES. After washing, the bead-immune complexes were suspended and rinsed with 100 µL of 1× SDS sample buffer, boiled for 5 min, and collected by brief centrifugation. To analyze the activation of mTORC2, the immunoprecipitates were subjected to immunoblot analysis with the phospho-mTOR (Ser2481) (1:1000; #651701; BioLegend, San Diego, CA) as the primary antibody. The blots were normalized by the abundance of mTOR (1:100; #2972 Cell Signaling Technology) in the immunoprecipitates.
Western blot
Total lysates (20–50 μg) and immunoprecipitated samples (10–40 μL) were resolved on 8 or 12% SDS‐polyacrylamide gel electrophoresis (PAGE) gels, followed by a transfer to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA).9 The membranes were then incubated in primary antibodies to the protein targets of interest, washed, and exposed to horseradish peroxidase-tagged secondary antibodies (1:10,000; #170-6515 and 170–6516; Bio-Rad, Hercules, CA). To normalize the phosphorylated target protein, blots were stripped in stripping buffer (# 46430; Pierce Biotechnology, Rockford, IL) and reprobed with housekeeping or non-phospho-antibodies. The primary antibodies that were used were: phospho-pan-/Akt (Ser472/473/474) (1:1000; #ab192623; Abcam, Cambridge, MA); pan-Akt, Akt1, Akt2, Akt3 (Rabbit mAb #4685, Rabbit mAb #75692, Rabbit mAb #3063, and Mouse mAb #8018), FAK (total, #3285 and Tyr397, #8556), mTOR (total, #2972), PTEN (total, #9552 and Ser308, #9551), PP2A (total, #2260) and PDK1 (total, #3062 and Ser241, #3438) (1:1000; Cell Signaling Technology); PP2A (Tyr307, # AF3989-SP; 1:1000; R&D System, Minneapolis, MN); Vinculin (#66305-1-lg; 1:4000); PHLPP (#676640-1-lg), Ubl4A (#14253-1-AP), IP6K1 (#12057-2-AP), Hsp90 (#13171-1-AP) and GAPDH (#60004-1-lg) (1:1000; Proteintech, Rosemont, IL); phospho-mTOR (Ser2481, #651701) (1:1000; BioLegend). The secondary antibodies were goat anti-mouse IgG (H + L)-HRP conjugate and goat anti-rabbit IgG (H + L)-HRP Conjugate (1:5000 or 1:10,000; #170-6515 and 170-6516; Bio-Rad).
Statistical analysis
Statistical analysis was performed with the mixed model procedure of SAS software (version 9.4; SAS Institute, Cary, NC). Two-factor analysis of variance (ANOVA) was used to analyze the main effects and interaction of gestational age at birth (preterm vs. term; GAB) and feeding state (fasted vs. fed; STATE) on the abundance and/or phosphorylation of proteins involved in the insulin signaling pathway; the pig was considered a random effect. Tukey’s post hoc test was used to assess differences among treatments when a significant interaction between main effects was detected. Data are presented as least-squares means ± standard error.
Results
Impact of preterm birth on plasma insulin concentrations and insulin signaling component activation and protein synthesis in the LD muscle
Circulating concentrations of insulin and the activation of insulin signaling components and fractional rates of protein synthesis in the LD muscle were previously published but are reported here for context.10 Circulating insulin concentrations were similar in preterm and term pigs during fasting conditions, rose more slowly in preterm compared to term pigs at 15 (46 ± 6 vs. 92 ± 8 μU/mL, respectively) and 30 (94 ± 7 vs. 122 ± 9 μU/mL, respectively) min after a meal (P < 0.05), but were similar by 45 (112 ± 8 vs. 114 ± 7 μU/mL, respectively) and 60 min after feeding (95 ± 9 vs. 89 ± 8 μU/mL, respectively). The activation of early steps of the insulin signaling pathway after a meal, i.e., the phosphorylation of the insulin receptor and IRS1, and the abundance of the IRS1-PI3K complex in the skeletal muscle were similar in preterm and term pigs. However, the phosphorylation of pan-Akt at Thr308 (0.46 ± 0.06 vs. 0.83 ± 0.16 AU) and Ser473 (0.30 ± 0.05 vs. 0.75 ± 0.05 AU) and the fractional protein synthesis rate in LD muscle (33.9 ± 1.2 vs. 42.9 ± 1.4 %/d, respectively) were lower in preterm compared to term pigs after a meal (P < 0.01).
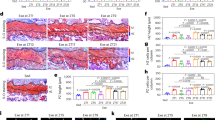
To further dissect the phosphorylation status of each Akt isoform, immunoprecipitation and immunoblotting techniques were performed. Compared to fasting, feeding induced the phosphorylation of all Akt isoforms (Akt1 Ser472, Akt2 Ser473, and Akt3 Ser474) in the LD muscle of both preterm and term pigs (P < 0.05; Fig. 2a–c). However, the feeding-induced phosphorylation of Akt1 and Akt2 (P < 0.01 for interaction between gestational age at birth/GAB and fasted vs fed/STATE), but not Akt3, was lower in preterm than in term pigs (P < 0.05).
The phosphorylation of individual Akt isoform was analyzed by immunoprecipitation followed by western blot. a–c Phosphorylated Akt1 at Ser472, Akt2 at Ser473, and Akt3 at Ser474. Phosphorylation values were corrected by their protein abundance in the immunoprecipitates. For Akt1 and Akt2, there was a significant interaction (P < 0.01) between GAB and STATE. Representative blots are shown. Data are expressed as mean ± SEM; n = 21–25/group (*P < 0.05 and **P < 0.01). AU arbitrary units.
Preterm birth blunts the activation of positive regulators of Akt
The phosphorylation of upstream and downstream positive regulators of Akt is crucial for maintaining Akt in an active state.15,16 Phosphorylation of PDK1 at Ser241 was induced by feeding in pigs born at term but not in preterm pigs (Fig. 3a; P < 0.05). There was a significant interaction (P < 0.001) between GAB and STATE. Phosphorylation of mTORC2 at Ser2481, which determines the activation status of mTORC2, was increased by feeding in both preterm and term pigs (Fig. 3b), but the response was lower in preterm than in term pigs (P < 0.05). In contrast, the phosphorylation of FAK, an activator of PI3K, was not affected by either feeding or preterm birth (Fig. 3c). The abundance of Ubl4A, an Akt-binding protein that is responsible for translocating Akt to the cell membrane, was not significantly affected by feeding but was lower in the muscle of preterm compared to term pigs (Fig. 3d; P < 0.05).
a Western analysis of phosphorylated PDK1 at Ser241. The phosphorylation of mTORC2, Akt-GAPDH complex abundance, and Akt-Hsp90 complex abundance were analyzed by immunoprecipitation followed by Western blot. b–f Western blot analysis of phosphorylated mTORC2 at Ser2481, phosphorylated FAK at Tyr397, Ubl4A abundance, Akt-GAPDH complex abundance, and Akt-Hsp90 complex abundance. Values were corrected by their protein abundance in the immunoprecipitates or tissue lysate samples. Ubl4A abundance values were normalized by GAPDH in the samples. For PDK1, there was a significant interaction (P < 0.001) between GAB and STATE. Representative blots are shown. Data are expressed as mean ± SEM; n = 21–25/group (*P < 0.05 and **P < 0.01). AU arbitrary units.
Depending on cell types, it has been shown that Akt can form complexes with its binding partners that can negatively or positively affect Akt activation.30 Using immunoprecipitation and immunoblotting assays, we determined the formation of Akt-GAPDH and Akt-Hsp90 complexes, which are positive regulators of Akt. As shown in Fig. 3e, f, protein–protein interaction assays showed that preterm birth reduced Akt-GAPDH and Akt-Hsp90 complex abundances, increasing exposure of Akt to the action of phosphatases (P < 0.05). However, in both preterm and term pigs, feeding did not affect the abundance of Akt-GAPDH and Akt-Hsp90 complexes.
Preterm birth enhances the activation of negative regulators of Akt
Although the inactivation of Akt by phosphatases and other inhibitors is crucial and part of normal homeostasis and cell functions, over- or under-activation of these inhibitors can be detrimental. Thus, we determined the abundance or phosphorylation of several major phosphatases and IP6K1. Preterm birth and feeding status did not affect PTEN activation (Fig. 4a). Phosphorylation of PP2A inhibits PP2A activity.23 Although premature pigs had more of the inactive form of PP2A, feeding reduced the abundance of the active PP2A form in term pigs, but not in preterm pigs (P < 0.05; GAB × STATE, P < 0.001; Fig. 4b). SHIP2 abundance, a lipid phosphatase that negatively regulate Akt activation, was greater in preterm than term piglets (Fig. 4c; P < 0.05). PHLPP directly dephosphorylates and inactivates Akt.31 Consistent with lower activation of Akt, the abundance of PHLPP was greater in preterm than term pigs, but PHLPP abundance was not affected by feeding (Fig. 4d; P < 0.05). Although IP6K1 has been known to be involved in producing IP7, a potent inhibitor of Akt,24 preterm birth did not affect IP6K1 abundance (Fig. 4e).
a–e Western analysis of phosphorylated PTEN at Ser380, phosphorylated PP2A at Tyr307, SHIP2, PHLPP, and IP6K1. Phosphorylation values were corrected by their protein abundance in the samples. Total protein abundance values were normalized either by GAPDH or vinculin abundances in the samples. For PP2A, there was a significant interaction (P < 0.001) between GAB and STATE. Representative blots are shown. Data are expressed as mean ± SEM; n = 21–25/group (*P < 0.05). AU arbitrary units.
Discussion
Despite improvements in the postnatal care and nutritional management of preterm infants, growth faltering among them is almost universal.32 One crucial aspect which may hinder efforts to improve growth outcomes in preterm infants is a lack of understanding of the molecular mechanisms by which nutrition regulates protein synthesis during the perinatal period. Recently, we showed that the feeding-induced stimulation of protein synthesis was lower in skeletal muscle of preterm compared to term piglets delivered by cesarean section and this was associated with a blunted activation of most signaling components involved in mTORC1-dependent activation of translation initiation.10 Using animal models, we and others showed that preterm birth reduced the feeding-induced activation of Akt without affecting the activation of the early steps of the insulin signaling pathway responsible for Akt activation, i.e., IR, IRS1, and PI3K.10,12 This suggests that preterm birth affects regulatory signaling components that are required to fully activate Akt. Therefore, in this study, we set out to search for potential upstream and downstream molecules that regulate Akt activation that are affected by preterm birth in the skeletal muscle of neonatal pigs.
Akt has emerged as a crucial kinase that affects diverse downstream signaling components involved in cellular metabolic processes including protein synthesis.33 We have shown previously that the activation of Akt after a meal is rapid in the neonate born at term, mirroring the time course of the change in circulating insulin, but the increase in protein synthesis is more sustained, reflecting stimulation by both insulin and amino acid signaling pathways.34 Akt consists of three isoforms (Akt1, Akt2, and Akt3) which are all expressed in skeletal muscle and have different physiological functions such as neural development (Akt3) and metabolism (Akt1 and Akt2).35,36 Previous studies, including our work, reported that preterm birth reduces the activation of Akt in skeletal muscle, however, its effect on the activation of Akt isoforms is unknown.10,12 In the current study, we used immunoprecipitation-immunoblotting approaches to delineate the effects of preterm birth on the activation of each Akt isoform. Our results demonstrate that activation of Akt1 and Akt2, but not Akt3, in response to feeding was lower in the skeletal muscle of preterm compared to term piglets, consistent with the reported function of Akt1 and Akt2 in cellular growth and glucose homeostasis.13
The regulation of Akt activation by its upstream and downstream effectors is complex and depends on numerous factors including different types of growth factors or mechanical stimuli and diverse cell types.37 One of the early steps of Akt activation is the translocation of Akt to the cell membrane by Ubl4A, a molecular chaperone, which induces Akt conformation change and subsequent activation by its kinases, PDK1 and mTORC2.19,37,38 The phosphorylation of Akt at residues Thr308 and Ser473 by PDK1 and mTORC2, respectively, is needed for full activation of Akt.37 Here we show the phosphorylation of both PDK1 and mTORC2 were significantly lower in preterm than in term piglets, indicating that preterm negatively affected the activation of these two major kinases. These data are also consistent with our previously reported data showing blunted postprandial Akt phosphorylation at Thr308 and Ser473 residues in the preterm.10 Ulb4A abundance is crucial for Akt activation at the plasma membrane.19 In this study, the observed reduction in Ulb4A abundance by preterm birth is consistent with the lower activation of Akt in the skeletal muscle of the preterm pig. FAK, another cytoplasmic tyrosine kinase, has also been shown to activate the Akt via the PI3K pathway that promotes cell growth.39 However, we were not able to detect any effect of preterm birth on the activation of FAK.
Another regulatory process that affects the activation state of Akt includes the interaction between Akt and its binding partners.30 An interaction of GAPDH, an enzyme in glycolysis, with Akt positively regulates Akt activation.25 The binding of Akt to Hsp90 can protect and maintain its kinase activity by preventing PP2A-mediated dephosphorylation.26,40 In this study, we found that the abundance of Akt-GAPDH and Akt-Hsp90 complexes was lower in the muscle of preterm than in term piglets, indicating that the phosphorylation status of Akt is less protected from phosphatase action in the skeletal muscle of the preterm. Taken together, the findings of our study suggest that the decreased Akt activation in skeletal muscle of the preterm in response to feeding is at least in part due to the attenuation of Akt translocation to the cell membrane, the activation of its kinases, and the abundance of the protein-protein complex formation between Akt and its binding partners.
The inactivation of Akt by phosphatases is critical for normal cellular homeostasis and cell growth. However, dysregulation of phosphatase activity can result in the hyper-activation of Akt, which is a common occurrence in cancer cells.27 PTEN is a major negative regulator that functions as a lipid phosphatase for PIP3, a crucial lipid secondary messenger formed by PI3K that activates PDK1.41 SHIP2 also acts as a lipid phosphatase that dephosphorylates the 5-phosphate of PIP3 and negatively regulates the PI3K/Akt pathway.42 Although we did not observe significant differences in PTEN activation, we found that the abundance of SHIP2 was higher in the skeletal muscle of preterm than in term piglets. Akt is also negatively regulated by two major classes of serine/threonine phosphatases, PP2A and PHLPP.31,43 More specifically, PP2A acts as a pivotal regulator controlling the dephosphorylation of Akt at Thr308 residues,43 while PHLPP is responsible for the dephosphorylation of Akt at Ser473 residues.31 Studies show that PP2A can be inactivated via multiple mechanisms, including the phosphorylation of PP2A at Tyr307 residues.44 In the current study, we found that the feeding-induced inactivation of PP2A was only present in the muscle of pigs born at term suggesting that PP2A is more resistant to inactivation by feeding in premature muscle. Suppressing PHLPP abundance using small interfering RNA greatly enhances the phosphorylation of Akt at Ser473 residues,31 and thus PHLPP abundance can serve as a bona fide readout of its activation.31 Our results demonstrate that PHLPP abundance in skeletal muscle was higher in preterm compared to term pigs, consistent with our finding that Akt phosphorylation at Ser473 residues was dampened by preterm birth.
A study using a genetic approach to determine the role of IP7, a newly identified inhibitor of Akt, revealed that IP6K1, a kinase that synthesizes IP7, an energy-rich small molecule, negatively affects Akt activation.24 Moreover, an in vivo study using a rodent model supports the notion that higher IP7 production by IP6K1 is associated with impaired Akt activation.45 In this study, we did not observe statistically significant differences in the abundance of IP6K1 in the muscle of preterm compared to term pigs suggesting that IP6K1 may not be a crucial regulator of Akt activation in neonates.
Premature birth remains a major factor that causes growth delay and stunting and that negatively affects health outcomes later in life. To support the lean growth of premature infants, it is essential to provide optimum nutrition to support an extrauterine growth rate that approximates the intrauterine growth rate of a healthy fetus at the same post-conception age, but remarkably little is known regarding the effects of preterm birth on the molecular mechanisms by which feeding stimulates skeletal muscle protein synthesis. In the present study, we addressed potential key players that regulate the feeding-induced activation of Akt, which in turn regulates protein synthesis via mTORC1 activation. Collectively, our study demonstrates that the feeding-induced activation of most of the positive regulators of Akt that we analyzed was reduced by preterm birth. Conversely, preterm birth induced the activation of several of the phosphatases that potently act as inhibitors of Akt activation in vivo. The preterm neonatal pig was used as a model of the human infant and the results of this study reflect the responses of the preterm in the extrauterine environment and may not reflect that which occurs in an unperturbed, physiologically stable fetal piglet. However, the birth mode was controlled for as both preterm and term pigs were delivered by cesarean section. Studies were conducted a few days after birth and further research is needed to determine whether these alternations in the preterm are sustained long term. Studies also are needed to apply this information to identify targets for improving the activation of Akt-mTORC1 signaling to protein synthesis in the skeletal muscle of preterm neonates to promote their lean growth.
Data availability
The datasets supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Barfield, W. D. Public health implications of very preterm birth. Clin. Perinatol. 45, 565–577 (2018).
Peila, C. et al. Extrauterine growth restriction: definitions and predictability of outcomes in a cohort of very low birth weight infants or preterm neonates. Nutrients 12, 1224–1234 (2020).
Platt, M. J. Outcomes in preterm infants. Public Health 128, 399–403 (2014).
Koontz, M. B., Gunzler, D. D., Presley, L. & Catalano, P. M. Longitudinal changes in infant body composition: association with childhood obesity. Pediatr. Obes. 9, e141–e144 (2014).
Kajantie, E., Osmond, C., Barker, D. J. & Eriksson, J. G. Preterm birth–a risk factor for type 2 diabetes? The Helsinki Birth Cohort Study. Diabetes Care. 33, 2623–2625 (2010).
Ahmad, I. Body composition and its components in preterm and term newborns: a cross-sectional, multimodal investigation. Am. J. Hum. Biol. 22, 69–75 (2010).
Davis, T. A. & Fiorotto, M. L. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. Metab. Care. 12, 78–85 (2009).
Davis, T. A. et al. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 282, E880–E890 (2002).
Suryawan, A. et al. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am. J. Physiol. Endocrinol. Metab. 293, E1597–E1605 (2007).
Naberhuis, J. K. et al. Prematurity blunts the feeding-induced stimulation of translation initiation signaling and protein synthesis in muscle of neonatal piglets. Am. J. Physiol. Endocrinol. Metab. 317, E839–E851 (2019).
Rudar, M. et al. Prematurity blunts the insulin- and amino acid-induced stimulation of translation initiation and protein synthesis in skeletal muscle of neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 320, E551–E565 (2021).
Blanco, C. L. et al. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology 156, 813–823 (2015).
Gonzalez, E. & McGraw, T. E. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8, 2502–2508 (2009).
Gao, Y., Moten, A. & Lin, H.-K. Akt: a new activation mechanism. Cell Res. 24, 785–786 (2014).
Lien, E. C., Dibble, C. C. & Toker, A. PI3K signaling in cancer: beyond AKT. Curr. Opin. Cell Biol. 45, 62–71 (2017).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Proc. Natl Acad. Sci. USA 97, 10832–10837 (2000).
Gupta, A. & Dey, C. S. PTEN and SHIP2 regulates PI3K/Akt pathway through focal adhesion kinase. Mol. Cell. Endocrinol. 309, 55–62 (2009).
Oudart, J. B. et al. The anti-tumor NC1 domain of collagen XIX inhibits the FAK/ PI3K/Akt/mTOR signaling pathway through alphavbeta3 integrin interaction. Oncotarget 7, 1516–1528 (2016).
Zhao, Y. et al. Ubl4A is required for insulin-induced Akt plasma membrane translocation through promotion of Arp2/3-dependent actin branching. Proc. Natl Acad. Sci. USA 112, 9644–9649 (2015).
Liao, Y. & Hung, M.-C. Physiological regulation of Akt activity and stability. Am. J. Transl. Res. 2, 19–42 (2010).
Carnero, A., Blanco-Aparicio, C., Renner, O., Link, W. & Leal, J. F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug. Targets 8, 187–198 (2008).
Azzi, A. SHIP2 inhibition alters redox-induced PI3K/Akt and MAP kinase pathways via PTEN over-activation in cervical cancer cells. FEBS Open Biol. 10, 2191–2205 (2020).
Seshacharyulu, P., Pandey, P., Datta, K. & Batra, S. K. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 335, 9–18 (2013).
Chakraborty, A. et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 97–910 (2010).
Jacquin, M. A. et al. GAPDH binds to active Akt, leading to Bcl-xL increase and escape from caspase-independent cell death. Cell Death Differ. 20, 1043–1054 (2015).
Sato, S., Fujita, N. & Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl Acad. Sci. USA 97, 10832–10837 (2000).
Zhang, Q. & Claret, F. X. Phosphatases: the new brakes for cancer development? Enzym. Res. 2012, 659649 (2012).
Burrin, D. G. et al. Translational advances in pediatric nutrition and gastroenterology: new insights from pig models. Ann. Rev. Anim. Biosci. 8, 321–335 (2020).
Williamson, D. L. Altered nutrient response of mTORC1 as a result of changes in REDD1 expression: effect of obesity vs. REDD1 deficiency. J. Appl. Physiol. 117, 246–256 (2014).
Du, K. & Tsichlis, P. N. Regulation of the Akt kinase by interacting proteins. Oncogene 24, 7401–7409 (2005).
Gao, T., Furnari, F. & Newton, A. C. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol. Cell 18, 13–24 (2005).
Hay, W. W. Jr Optimizing nutrition of the preterm infant. Zhongguo Dang Dai Er Ke Za Zhi 19, 1–21 (2017).
Glass, D. J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 346, 267–278 (2010).
Wilson, F. A. et al. Feeding rapidly stimulates protein synthesis in skeletal muscle of the neonatal pig by enhancing translation initiation. J. Nutr. 139, 1873–1880 (2009).
Hers, I., Vincent, E. E. & Tavaré, J. M. Akt signalling in health and disease. Cell Signal. 23, 1515–1527 (2011).
Matheny, R. W. Jr. et al. Akt2 is the predominant Akt isoform expressed in human skeletal muscle. Physiol. Rep. 6, 1–8 (2018).
Manning, B. D. & Alex Toker, A. Akt/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Toker, A. & Cantley, L. C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387, 673–676 (1997).
Paul, R. et al. FAK activates Akt-mTOR signaling to promote the growth and progression of MMTV-Wnt1-driven basal-like mammary tumors. Breast Cancer Res. 22, 59–73 (2020).
Yun, B.-G. & Matts, R. L. Hsp90 functions to balance the phosphorylation state of Akt during C2C12 myoblast differentiation. Cell Signal. 17, 1477–1485 (2005).
Georgescu, M. M. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer 1, 1170–1177 (2010).
Pan, Q., Wu, J., Liu, Y., Li, X. & Chen, J. Involvement of hepatic SHIP2 and PI3K/Akt signalling in the regulation of plasma insulin by xiaoyaosan in chronic immobilization-stressed rats. Molecules 24, 480–496 (2019).
Millward, T. A., Zolnierowicz, S. & Hemmings, B. A. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24, 186–191 (1999).
Sangodkar, J. et al. All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase. FEBS J. 283, 1004–1024 (2016).
Zhang, Z. et al. Inositol pyrophosphates mediate the effects of aging on bone marrow mesenchymal stem cells by inhibiting Akt signaling. Stem Cell Res. Ther. 5, 33–45 (2014).
Acknowledgements
This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does the mention of trade names, commercial products, or organization imply endorsement by the US government. We thank H.V. Nguyen and R.D. Parada for expert technical assistance and the staff of the Comparative Nutrition Research Facility for animal care.
Funding
This work was supported by the National Institute of Child Health and Human Development Grants HD-085573 (T.A.D.), HD-072891 (T.A.D.), HD-099080 (T.A.D. and M.L.F.), USDA Current Research Information System Grant 3092-51000-060 (T.A.D.), and USDA National Institute of Food and Agriculture Grant 2013-67015-20438 (T.A.D.).
Author information
Authors and Affiliations
Contributions
A.S., M.L.F., and T.A.D. conceived and designed the research; A.S., M.R., and J.K.N. performed experiments; A.S. and M.R. analyzed data; A.S., M.R., J.K.N., and M.L.F. interpreted the results of experiments; A.S. drafted the manuscript; A.S., M.R., J.K.N., M.L.F., and T.A.D. edited and revised the manuscript; A.S., M.R., J.K.N., M.L.F., and T.A.D. approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suryawan, A., Rudar, M., Naberhuis, J.K. et al. Preterm birth alters the feeding-induced activation of Akt signaling in the muscle of neonatal piglets. Pediatr Res 93, 1891–1898 (2023). https://doi.org/10.1038/s41390-022-02382-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02382-4