Abstract
Direct oral anticoagulants (DOACs) are widely used to treat venous thromboembolism (VTE) in adults. Little attention is given to pediatric VTE (PVTE). The objective of this study is to study the efficacy and safety of DOACs in published PVTE randomized control trials (RCTs). PubMed, Embase, China National Knowledge Infrastructure, the Cochrane Library, SinoMed, and ClinicalTrials.gov were searched until 2021, to identify RCTs that enrolled patients with VTE <18 years of age who received DOACs versus standard anticoagulation. Outcomes were evaluated using the Mantel–Haenszel method of random-effects model. Our study evaluated seven RCTs that included 1139 cases of PVTE, which had a low risk of publication and assessment bias. Compared with standard anticoagulation, patients receiving DOACs presented a lower rate of recurrent VTE (relative risk [RR], 0.42 [confidence interval {CI}, 0.20 to 0.89]), similar mortality rate (RR, 0.50 [CI, 0.07 to 3.57]), major bleeding (RR, 0.46 [CI, 0.14 to 1.57]), and higher clinically relevant nonmajor bleeding (RR, 2.71 [CI, 1.05 to 7.02]) with low heterogeneity. Limiting to subgroups, dabigatran and rivaroxaban yielded similar findings, except for a higher incidence of nonmajor bleeding during rivaroxaban use. DOACs could be an alternative to standard anticoagulation in PVTE. Dabigatran and rivaroxaban have similar effects.
Impact
-
In venous thromboembolism (VTE), direct oral anticoagulants (DOACs) are widely used as a substitution for standard anticoagulation in most situations for adults; however, little attention is paid to the pediatric population.
-
For pediatric VTE, previous meta-analyses have emphasized the epidemiology, risk factors, and the use of traditional anticoagulants, and seldom reported the use of novel oral anticoagulants.
-
This is the first meta-analysis of randomized controlled trials that focuses on the efficacy outcomes and safety endpoints of DOACs compared with standard anticoagulation in pediatric VTE.
Similar content being viewed by others
Introduction
With advances in diagnostic procedures and treatment in modern medicine, the prevalence of venous thromboembolism (VTE) in the pediatric population has been accelerating sharply. A 70% annual rise in the number of patients with VTE per 10,000 children in America has been reported.1,2,3 Pediatric VTE (PVTE) is becoming a concerning situation; therefore, there is an extremely urgent need for a reasonable and normative anticoagulation strategy.
Monitoring anticoagulant therapy to achieve outcomes within the target range is the standard treatment in childhood VTE. However, parenteral administration of medications and frequent blood tests in children is often a cumbersome process.4,5 Treatment using vitamin K antagonists, an approach for long-term standard anticoagulation (SAC), has several shortcomings: the absorption of these compounds is easily affected by food, medications, and dosage adjustments, and is associated with poor compliance.6 All these factors lead to the implementation of SAC for PVTE extremely difficult, especially for those requiring prolonged anticoagulation. Thus, identifying a substitute for SAC for children who can be administered orally and without a regular blood test appears to be particularly valuable.
Direct oral anticoagulants (DOACs), including rivaroxaban, edoxaban, apixaban, and dabigatran, do not require routine monitoring during treatment and are widely used to manage conditions, such as lower extremity venous thrombosis, pulmonary embolism, visceral venous thrombosis, thrombotic sequelae, and after vena cava filter implantation and as secondary prevention.7 Furthermore, the United States (US) Food and Drug Administration, the European Medicines Agency, and Health Canada have approved edoxaban, apixaban, dabigatran, and rivaroxaban as alternative drugs for VTE to reduce the risk of recurrence or cancer-related VTE in adults;8 however, none of them had recommended guidelines specifically for children until now. Children are inherently vulnerable to thromboembolic events that are difficult to monitor, and have a greater risk of experiencing adverse events resulting in unpredictable outcomes.9 These factors led physicians to consciously choose a safe and effective anticoagulant drug to treat children with VTE. Could DOACs be used as a substitute for SAC in treating VTE in pediatric patients?
Currently, clinical practice indications for anticoagulation in pediatric patients with VTE are largely extrapolated from data obtained from trials in adults.6,10 The anatomic distribution, pathophysiology of thrombosis, and level of hemostatic proteins in children are significantly different from those in adults.9 Little attention is given to the possibility of whether DOACs can be suitable for PVTE. Practically, hematologists in hemophilia treatment centers have already treated PVTE with DOACs, although there are no official guidelines recommended in the US.11,12 In addition, DOACs used to treat PVTE have been documented in some published articles, conferences, and even ongoing trials (e.g., NCT02369653, NCT02464969, NCT02303431). In recent years, randomized controlled trials (RCTs) on DOACs vs. SAC in children with VTE are emerging. The Einstein Junior (NCT02234843) study compared oral rivaroxaban to SAC in PVTE based on a large RCT and demonstrated that the rivaroxaban group had a similar recurrence of VTE and bleeding risk compared with SAC. Another trial (the Diversity trial, NCT01895777) that evaluated dabigatran in pediatric patients found no distinct differences in the major outcomes between the use of dabigatran and SAC. The data from these clinical trials provide a strong foundation for the practice of using DOACs to treat PVTE or as secondary prophylaxis and indicate its potential for use in a clinical setting.13,14,15 The aim of our meta-analysis was to investigate the efficacy and safety of DOACs versus SAC in children with VTE. The protocol is available at PROSPERO (CRD42021286154).
Methods
Search strategy
PubMed, Embase, China National Knowledge Infrastructure, the Cochrane Library, and SinoMed were searched for RCTs that enrolled subjects younger than 18 years of age with VTE, wherein the efficacy of DOACs versus SAC was compared (until October 18, 2021). ClinicalTrials.gov was also searched to identify complete RCTs that had results. SAC therapy includes the use of heparin, low-molecular-weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, and enoxaparin/vitamin K antagonists for a certain period. The search strategy is depicted in the Supplementary text File. Only RCTs that enrolled participants with VTE aged 18 years or younger, and that compared DOACs versus SAC therapy in these participants were included. Duplicate studies, meta-analyses, reviews, letters, and commentaries were not considered. Moreover, single-arm studies, phase 2 studies, studies with inconsistent outcomes, and clinical trials that did not report complete results were excluded from this analysis. All references were downloaded for further analysis.
Eligibility criteria
Eligibility criteria for this study were laid down by two of the authors (J.C. and G.S.B.) and amended by the co-authors. Two blinded authors (J.C. and G.S.B.) independently selected studies and abstracted data based on the inclusion and exclusion criteria. Discrepancies were resolved via a discussion by J.C., G.S.B., and F.W., and finally decided by X.Q. The eligibility criteria were as follows: (1) patients younger than 18 years of age who were receiving treatment for VTE; (2) RCTs; and (3) studies that recorded efficacy outcomes or safety endpoints of the comparison of DOACs and SAC.
Studies without detailed data or those in which the outcome indicators were inconsistent were excluded.
The efficacy outcomes comprised recurrent VTE and VTE-related mortality. The safety endpoints included major bleeding and clinically relevant nonmajor bleeding (CRNMB). The time of follow-up was also abstracted from the original studies. The definitions of VTE, VTE-related mortality, major bleeding, and CRNMB were identified based on the references.16,17
Risk of bias assessment
Bias among studies including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias was assessed using the Cochrane Risk Assessment Form.18 Modified Jadad assessment was used for evaluation of the quality of methodology,19 scoring different degrees of randomization, concealment of allocation, double blinding, and withdrawals and dropouts. Lastly, the total score was calculated, which could help with the identification and assessment of methodology quality (low or high).
Statistical analysis
The Mantel–Haenszel method of random-effects model was used to analyze groups. Outcomes were calculated using relative risks (RRs) and 95% confidence intervals (95% CIs). Heterogeneity was identified by adopting the I2 parameter, wherein low I2 was defined as <25%, moderate as I2 < 50%, and high as I2 > 50%.20 When I2 value was >50%, the random-effects model was used; if not, the fixed-effects model was used. Funnel plots and forest plots were used to evaluate publication bias by measuring the total primary and safety outcomes and subgroups. Sensitivity analyses were conducted to evaluate the contribution of each study. RevMan version 5.2 was used for the meta-analysis.
Results
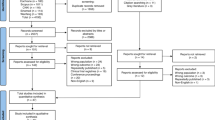
Our initial search identified 137 potentially relevant publications from databases and 5 RCTs after searching the Cochrane database and ClinicalTrials.gov. Titles and abstracts were screened, and duplicate and irrelevant references, letters, reviews, studies with inconsistent outcomes, those without results, and single-arm trials were excluded. Lastly, seven studies (five articles and two completed clinical trials) were selected. Among them, five studies compared rivaroxaban with SAC, and two studies compared dabigatran with SAC (Fig. 1). The seven studies included a total of 1139 patients with VTE (769 treated with DOACs and 370 treated with SAC) from multicenter and multicountry multicenter, except Dabigatran trial (Aziz Eghbali et al., 2020) only from Iran. The primary outcomes (recurrent VTE and mortality) and safety endpoint outcomes (major bleeding and CRNMB) were collected in these groups. The brief overall characteristics of the seven RCTs were recorded and are presented in Table 1. The seven studies included in this meta-analysis were completed or published during the years 2018–2021.
Treatment dosage in studies
Initially, patients in the groups were treated for several days with UFH, LMWH, or enoxaparin, followed by dabigatran or rivaroxaban. The dose of dabigatran was determined after being adjusted for age and weight according to the protocol by Hayton.21,22,23 In the included studies, patients in the rivaroxaban groups were given a body weight-adjusted dose of 20 mg, either 1, 2, or 3 times a day in the form of capsules, pellets, or oral solution, which was determined based on age or weight. The SAC groups continued treatment with LMWHs, UFH, fondaparinux, or switched to vitamin K antagonists, at the dosages based on the investigators’ judgment and standard clinical practices (Table 1).
Risk of bias
Cochrane risk of bias assessment and modified Jadad quality evaluation were used for all RCTs. All trials were open-label and randomized. A blinded, independent organization was employed to collect data to assure outcomes. Thus, the assessment had a low risk of selection bias. Performance bias was a high risk for all RCTs, as treatment behaviors could not be blinded to all participants, especially physicians. The remaining evaluation bias in the Cochrane risk was low and did not show any variation (Supplementary Fig. 1). The modified Jadad quality evaluation indicated that the meta-analyses of RCTs were of high quality in terms of their methodology (Supplementary Table 1). For comparisons involving seven studies, visual inspection revealed the Begg funnel plots for studies of primary efficacies and principal safety outcomes to be symmetrical (Supplementary Figs. 2 and 3). Forest plots used to analyze these events revealed no significant publication bias (Supplementary Figs. 4 and 5).
Recurrent VTE (RVTE)
Data from the NCT02309411 and NCT01684423 trials were excluded as RVTE data were not reported. This may have been mainly due to the limited 30- or 60-day follow-up. The remaining five studies reported RVTE as an outcome (11 events occurred in 686 patients during treatment with DOACs and 15 events occurred in 344 children during treatment with SAC). The pooled data showed that the use of DOACs could reduce the risk of RVTE (RR, 0.42 [95% CI, 0.20 to 0.89]; I2 = 0%) (Fig. 2). Analyses of the subgroup of rivaroxaban showed that there were no differences in reducing RVTE compared with SAC (RR, 0.35 [95% CI, 0.11 to 1.18]; I2 = 0%). Similar results were obtained in the analysis of the dabigatran subgroup (RR, 0.45 [95% CI, 0.18 to 1.21]; I2 = 0%). The risk-difference model in these five trials indicated similar results (absolute risk difference, –0.03 [95% CI, –0.05 to –0.00]).
VTE-related mortality
The data of VTE-related mortality were available for six studies (the event of death was not recorded in NCT01684423). Compared with that in children who received SAC, the VTE-related mortality in those who received DOACs was not significantly different (RR, 0.50 [95% CI, 0.07 to 3.57]; I2 = 0%) (Fig. 3). Analysis of mortality data in subgroups revealed that there were no significant differences between receiving DOACs and SAC in the rivaroxaban group (RR, 1.48 [95% CI, 0.06 to 36.19]) or the dabigatran group (RR, 0.17 [95% CI, 0.01 to 4.14]; I2 was not applicable because of only one child who received SAC died, 2021, dabigatran). Risk-difference analysis was performed and the results were in agreement with the RR (risk difference, 0.00 [95% CI, –0.01 to 0.01]).
Major bleeding
Seven studies reported information on major bleeding (4 in 769 patients receiving DOACs and 6 in 370 controls). The pooled risk of major bleeding in PVTE treated with DOACs was not statically significantly different from that treated with SAC (RR, 0.46 [95% CI, 0.14 to 1.57]; I2 = 0%) (Fig. 4). When the results of major bleeding in subgroups were analyzed, the findings were found to be similar (rivaroxaban, RR, 0.13 [95% CI, 0.02 to 1.21]; I2 = 0%; dabigatran, RR, 0.8 [95% CI, 0.18 to 3.52]; I2 = 0%) and was the same as risk difference (risk difference, –0.01 [95% CI, –0.02 to 0.01]; I2 = 0%).
CRNMB
With the exception of NCT02309411 studies did not provide details on CRNMB, only the presence of anemia was mentioned, if at all. Among the RCTs, six studies reported information on CRNMB (25 events in 729 patients receiving DOACs and 4 in 364 comparators). The risk of CRNMB in patients receiving DOACs and comparators was higher in the DOACs group than those in the SAC group (RR, 2.71 [95% CI, 1.05 to 7.02]; I2 = 0%) (Fig. 5). When the analysis was limited to rivaroxaban, minor higher risk of CRNMB was identified in children receiving rivaroxaban compared with the comparators (RR, 4.61 [95% CI, 1.27 to 16.72]; I2 = 0%). However, when only the outcome of dabigatran was assessed, there was no evidence of an increased risk of CRNMB between patients receiving dabigatran and those receiving SAC (RR, 0.74 [95% CI: 0.15 to 3.73]; I2 = 0%). The risk difference model in the six trials indicated no significant differences (risk difference, 0.02 [95% CI, 0.01 to 0.04]).
Discussion
The objectives of this meta-analysis were to investigate the efficacy outcomes and safety endpoints of DOACs. The pooled evidence demonstrated that DOACs were slightly superior to SAC as a whole and exhibited improved efficacy in preventing RVTE, while enabling easier access to treat CRNMB in the population of children with VTE. Dabigatran was found to be noninferior to SAC; however, patients in the rivaroxaban group showed a significantly higher incidence of CRNMB events in our study. These findings were consistent with a previous network meta-analysis of patients with atrial fibrillation that compared rivaroxaban with dabigatran or warfarin in adults.24
There has been a steady increase in the number of children with VTE. The management of children with this condition is challenging. On one hand, the incidence of venous thrombosis in the pediatric population is increasing every year mainly due to improvements and advances in interventional therapy. For example, the placement of a central venous catheter or a peripherally inserted central catheter can be lifesaving in many critically ill children, but can also lead to catheter-related thrombosis.25,26 Without a doubt, advances in modern medical science have helped decrease mortality, improve the rescue rate of critically ill children, and have been effective in preventing severe infections in children and treating hematological tumors that are often associated with venous thrombosis.27,28 Failure in the timely identification and effective treatment of VTE in children may lead to pulmonary embolism, post-thrombotic syndrome, several adverse effects, and serious life-threatening conditions.29,30 On the other hand, the effective and safe use of anticoagulant drugs to treat PVTE is also another crucial matter to address. Presently, there are no specialized versions of the vena cava filter or the mechanical thrombus-removal device for children, the main strategy to treat venous thrombosis in children is by using anticoagulant drugs.31 In the real world, the classical treatment of PVTE is by using SAC as recommended in the guidelines, but its implementation is met with challenges due to difficulties in patient surveillance.9 Physicians have been trying to identify new, safe and effective anticoagulants for PVTE. In the adult population, experts have paid more attention to oral anticoagulants, several related meta-analyses have identified the safety and efficacy of this intervention in adults with VTE receiving DOACs, including the elderly. The results show that compared with vitamin K antagonists, DOACs are capable of reducing the risk for RVTE and VTE-related mortality with no differences in major bleeding and CRNMB.32,33,34 DOACs have been widely used to treat adult VTE owing to their prominent advantages. However, could it be also a prospective class of anticoagulants for PVTE? Unlike adults, pediatric patients are more fragile and susceptible to various drugs used to treat hemostasis and coagulation disorders, the drug pharmacology and pharmacokinetics also differ in the pediatric population.10,23 The current dosing and management recommendations for children largely follow those recommended for adults. On account of no authority-approved PVTE oral anticoagulants, clinicians are actively trying to identify strategies and clinical trials of DOACs in PVTE.
In the case of PVTE, previously published meta-analyses have always emphasized the epidemiology, risk factors, second prevention, and the use of traditional anticoagulants, while seldom considering the use of novel oral anticoagulants.32,35,36,37,38 Only a few studies have reported the latest measures for treating venous thrombosis in children using interventions such as DOACs, which, are difficult to implement pediatric ethical considerations and the shortcomings of pediatric medications.6 Of late, more attention is being paid to DOACs for the treatment of PVTE. A single-arm prospective cohort trial among 3-month-old to 18-year-old patients suggests that children benefit from secondary VTE prevention by using dabigatran compared with SAC.39 The phase II trials of the four DOACs have been completed, and single-arm studies have also shown to be safe and efficacious in children.40,41,42,43,44,45 In this study, seven included RCTs served as a high-quality assessment tool to ensure the reliability of DOACs for PVTE, and the results indicated negligible heterogeneities. Noticeably, our analyses of both groups and subgroups comparing DOACs with SAC indicated encouraging outcomes, especially for dabigatran, which appeared to be prioritized for children with VTE who need long-term anticoagulation. It comes to a latest published review in Lancet Child Adolescent Health, which points out that DOACs could serve as a substitute for children and may be safer.46 Another systematic review conducted by Branstetter also indicated that the use of novel oral anticoagulants in PVTE was a safe and effective method, although only two clinical trials have focused on it.47 Moreover, these trials have not provided statistical data analysis. It is worth mentioning that this is the first meta-analysis of RCTs that focuses on the efficacy outcomes and safety endpoints of DOACs in PVTE with RCTs involved.
Our meta-analysis has several potential clinical implications. First, seven RCTs were enrolled in this study that included 1139 participants from worldwide involved. Compared with previous studies, as a condition that does not have a high-incidence disease, several patients across multiple geographical regions were investigated from the viewpoint of treating PVTE, thereby making the results more reliable. In addition, previous studies on PVTE have mainly focused on the treatment using traditional anticoagulation. This study is the first meta-analysis to compare the safety and efficacy of DOACs with SAC by using novel oral anticoagulants in children with VTE. Similar to the meta-analysis of studies that focus on treatment of VTE in adults,48 our study also confirmed that novel oral anticoagulants are noninferior to traditional anticoagulants for PVTE, except for an increase in CRNMB. Our study provides more options for physicians to make clinical decisions when treating PVTE. For example, dabigatran can be a better choice for children requiring long-term anticoagulation. The findings from our study will motivate clinicians to design and conduct larger clinical studies to generate more evidence.
Our meta-analysis still has some limitations. Firstly, due to practical and ethical difficulties in conducting clinical trials on children, we could only collect information from seven RCTs. The small list of included studies was consistent with clinical heterogeneity and introduced limitations to the pooled results, and the outcomes may differ in larger studies. Thus, the findings from our study should be further verified based on more RCTs. Secondly, incomplete primary outcomes, the lack of detailed individual data, and ambiguous descriptions may have potentially biased the results and affected the pooled estimates. Two completed clinical trials did not present RVTE events and details of CRNMB. Moreover, important adverse events such as pulmonary embolism and post-thrombotic syndrome were missed or not systematically revealed in the included studies, which led to limitations in the analysis. Therefore, further studies are needed to explore these crucial outcomes. Thirdly, the follow-up period was not consistent and ranged anywhere from 30 to 91 days. Varying follow-up duration may contribute to the heterogeneity observed in the RVTE. This could be an important reason why a 30-day study duration is insufficient for evaluating the recurrence of VTE in two of the clinical trials. In addition, only a few available studies could be incorporated, and the safety and efficiency of all DOACs could not be assessed due to a lack of trials on apixaban and edoxaban. Future trials that directly compare the efficacy of the four DOACs might support the robustness of our findings.
Conclusions
RCTs were included in this meta-analysis to prove the efficacy outcomes and safety endpoints of DOACs. Despite the limitations of our study, our findings indicated that compared with SAC, the pediatric population with VTE who received DOACs had a lower rate of RVTE, a similar rate of VTE-related mortality and major bleeding, and a higher incidence of CRNMB. Thus, opting for DOACs for a selected population of children could be a viable alternative. This analysis also revealed that dabigatran and rivaroxaban were noninferior to SAC except that rivaroxaban had a higher incidence of CRNMB events. Other DOACs should be evaluated in larger trials to confirm their benefits and harms and to further verify our results.
Data availability
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request. All data generated or analyzed during this study are included in the article.
References
Raffini, L., Huang, Y. S., Witmer, C. & Feudtner, C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics 124, 1001–1008 (2009).
Brown, M. A. & Fulkerson, D. H. Incidence of venous thromboembolism in hospitalized pediatric neurosurgical patients: a retrospective 25-year institutional experience. Childs Nerv. Syst. 36, 987–992 (2020).
Sharathkumar, A. A. et al. Epidemiology and outcomes of clinically unsuspected venous thromboembolism in children: a systematic review. J. Thromb. Haemost. 18, 1100–1112 (2020).
Bidlingmaier, C. et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta-analysis of single-arm studies. Semin Thromb. Hemost. 37, 814–825 (2011).
Monagle, P. & Newall, F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematol. Am. Soc. Hematol. Educ. Program 2018, 399–404 (2018).
Goldenberg, N. A. et al. Improving evidence on anticoagulant therapies for venous thromboembolism in children: key challenges and opportunities. Blood 126, 2541–2547 (2015).
Chopard, R., Albertsen, I. E., & Piazza, G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA 324, 1765–1776 (2020).
Renner, E. & Barnes, G. D. Antithrombotic management of venous thromboembolism: JACC Focus Seminar. J. Am. Coll. Cardiol. 76, 2142–2154 (2020).
Macartney, C. A. & Chan, A. K. Thrombosis in children. Semin Thromb. Hemost. 37, 763–761 (2011).
Monagle, P. et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2, 3292–3316 (2018).
Davila, J. G. et al. Characterizing the use of direct oral anticoagulants in children using the American Thrombosis and Hemostasis Network Dataset (ATHNdataset). Blood 134, 1156 (2019).
Davila, J. G. et al. Athn 15: characterizing the real-world use of direct oral anticoagulants in pediatric patients – interim analysis. Blood 136, 19-20 (2020).
Kucine, N. et al. Provider practices regarding prophylactic anticoagulation in children with leukemia. Blood 134, 3412 (2019).
Pinchinat, A. et al. A pilot study of an oral anticoagulant, apixaban, in secondary prophylaxis of venous thromboembolism (VTE) in children and adolescents. Blood 134, 4 (2019).
Manis, M. M., Cummins, L. E., Kyle, J. A. & Taylor, S. M. Successful use of apixaban for Paget-Schroetter syndrome in a pediatric patient. J. Pediatr. Pharm. Ther. 26, 508–511 (2021).
Schulman, S. & Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 3, 692–694 (2005).
Kaatz, S. et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J. Thromb. Haemost. 13, 2119–2126 (2015).
Corbett, M. S., Higgins, J. P. & Woolacott, N. F. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth. Methods 5, 79–85 (2014).
Palys, K. E. & Berger, V. W. A note on the jadad score as an efficient tool for measuring trial quality. J. Gastrointest. Surg. 17, 1170–1171 (2013).
Kahan, B. C. & Harhay, M. O. Many multicenter trials had few events per center, requiring analysis via random-effects models or GEEs. J. Clin. Epidemiol. 68, 1504–1511 (2015).
Chalmers, E. et al. Guideline on the investigation, management and prevention of venous thrombosis in children. Br. J. Haematol. 154, 196–207 (2011).
Lebas, A. et al. EPNS/SFNP guideline on the anticoagulant treatment of cerebral sinovenous thrombosis in children and neonates. Eur. J. Paediatr. Neurol. 16, 219–228 (2012).
Monagle, P. et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141, e737S–e801S (2012).
Bai, Y., Deng, H., Shantsila, A. & Lip, G. Rivaroxaban versus dabigatran or warfarin in real-world studies of stroke prevention in atrial fibrillation: systematic review and meta-analysis. Stroke 48, 970–976 (2017).
Shah, S. H. et al. Clinical risk factors for central line-associated venous thrombosis in children. Front Pediatr. 3, 35 (2015).
Patel, N. et al. Rates of venous thromboembolism and central line-associated bloodstream infections among types of central venous access devices in critically ill children. Crit. Care Med. 48, 1340–1348 (2020).
Onyeama, S. N. et al. Central venous catheter-associated venous thromboembolism in children with hematologic malignancy. J. Pediatr. Hematol. Oncol. 40, e519–e524 (2018).
Mitchell, W. B. et al. Children and young adults hospitalized for severe COVID-19 exhibit thrombotic coagulopathy. Pediatr. Blood Cancer 68, e28975 (2021).
Betensky, M. & Goldenberg, N. A. Post-thrombotic syndrome in children. Thromb. Res. 164, 129–135 (2018).
Carpenter, S. L., Richardson, T. & Hall, M. Increasing rate of pulmonary embolism diagnosed in hospitalized children in the United States from 2001 to 2014. Blood Adv. 2, 1403–1408 (2018).
Blevins, E. M. et al. A multicenter cohort study of inferior vena cava filter use in children. Pediatr. Blood Cancer 62, 2089–2093 (2015).
Neshat-Vahid, S. et al. Association of thrombophilia and catheter-associated thrombosis in children: a systematic review and meta-analysis. J. Thromb. Haemost. 14, 1749–1758 (2016).
Mai, V. et al. Extended anticoagulation for VTE: a systematic review and meta-analysis. Chest 155, 1199–1216 (2019).
Chaudhary, R. et al. DOACs versus VKAs in older adults treated for acute venous thromboembolism: systematic review and meta-analysis. J. Am. Geriatr. Soc. 68, 2021–2026 (2020).
Schoot, R. A., Kremer, L. C., van de Wetering, M. D. & van Ommen, C. H. Systemic treatments for the prevention of venous thrombo-embolic events in paediatric cancer patients with tunnelled central venous catheters. Cochrane Database Syst. Rev. 9, Cd009160 (2013).
Mahajerin, A. et al. Hospital-associated venous thromboembolism in pediatrics: a systematic review and meta-analysis of risk factors and risk-assessment models. Haematologica 100, 1045–1050 (2015).
Klaassen, I. L. M. et al. Are low-molecular-weight heparins safe and effective in children? A systematic review. Blood Rev. 33, 33–42 (2019).
Engel, E. R. et al. Predictors of postthrombotic syndrome in pediatric thrombosis: a systematic review and meta-analysis of the literature. J. Thromb. Haemost. 18, 2601–2612 (2020).
Brandão, L. R. et al. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood 135, 491–504 (2020).
Yee, D. L., O'Brien, S. H. & Young, G. Pharmacokinetics and pharmacodynamics of anticoagulants in paediatric patients. Clin. Pharmacokinet. 52, 967–980 (2013).
Halton, J. M. L. et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of dabigatran etexilate oral liquid formulation in infants with venous thromboembolism. Thromb. Haemost. 117, 2168–2175 (2017).
Yetman, R. J. et al. Apixaban pharmacodynamic activity in umbilical cord, paediatric, and adult plasma. Thromb. Haemost. 117, 1518–1527 (2017).
Monagle, P. et al. Bodyweight-adjusted rivaroxaban for children with venous thromboembolism (EINSTEIN-Jr): results from three multicentre, single-arm, phase 2 studies. Lancet Haematol. 6, e500–e509 (2019).
Sinegre, T. et al. In vitro assessment of edoxaban anticoagulant effect in pediatric plasma. Thromb. Res. 178, 112–118 (2019).
Esch, J. J., Hellinger, A., Friedman, K. G. & VanderPluym, C. J. Apixaban for treatment of intracardiac thrombosis in children with congenital heart disease. Interact. Cardiovasc Thorac. Surg. 30, 950–951 (2020).
Jaffray, J. & Young, G. Direct oral anticoagulants for use in paediatrics. Lancet Child Adolesc. Health 6, 207–214 (2022).
Branstetter, J. et al. Efficacy and safety of non-vitamin K antagonist oral anticoagulants in pediatric venous thromboembolism treatment and thromboprophylaxis: a systematic review of the literature. Semin. Thrombosis Hemost. 47, 643–653 (2021).
Ng, C. et al. A network meta-analysis of direct oral anticoagulants for portal vein thrombosis in cirrhosis. Hepatol. Int. 15, 1196–1206 (2021).
Funding
Project of Hunan Provincial Health Commission (B2019111). Hunan Provincial Natural Science Foundation Project (2020JJ5504, 2022JJ40385); Guangxi Science and Technology Planning Project (2017AB45033).
Author information
Authors and Affiliations
Contributions
Conception and study design: J.C., G.S.B., X.Q.; data search and extraction: J.C., F.W.; data analysis: J.C., G.S.B., F.W.; manuscript revision: X.Q. All authors were involved in writing the paper, and in reading and approving the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Bi, G., Wu, F. et al. Direct oral anticoagulants versus standard anticoagulation in children treated for acute venous thromboembolism. Pediatr Res 93, 1491–1498 (2023). https://doi.org/10.1038/s41390-022-02294-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02294-3
This article is cited by
-
Comparison of the efficacy and safety between rivaroxaban and dabigatran in the treatment of acute portal vein thrombosis in cirrhosis
BMC Gastroenterology (2023)
-
Efficacy and Safety of Direct Oral Anticoagulants in Pediatric Venous Thromboembolism: A Systematic Review and Meta-Analysis
Indian Journal of Pediatrics (2023)