Abstract
Background
Nasal continuous positive airway pressure, nasal intermittent positive pressure ventilation, and non-invasive neurally adjusted ventilatory assist are modes of non-invasive respiratory support. The objective was to investigate if cardiorespiratory measures performed shortly after extubation are associated with extubation outcomes and predictors of extubation success.
Methods
Randomized crossover trial of infants with birth weight (BW) ≤ 1250 g undergoing their first extubation. Shortly after extubation, electrocardiogram and electrical activity of the diaphragm (Edi) were recorded during 40 min on each mode. Measures of heart rate variability (HRV), diaphragmatic activity (Edi area, breath area and amplitude), and respiratory variability (RV) were computed on each mode and compared between infants with extubation success or failure (reintubation ≤ 7 days).
Results
Twenty-three extremely preterm infants with median [IQR] gestational age 25.9 weeks [25.2–26.4] and BW 760 g [595–900] were included: 14 success and 9 failures. There were significant differences for HRV (very low-frequency power and sample entropy) and RV parameters (breath areas, amplitudes and expiratory times) between groups, with moderate strength (0.75–0.80 areas under ROC curves) in predicting success. Diaphragmatic activity measures were similar between groups.
Conclusions
In extremely preterm infants receiving non-invasive respiratory support shortly after extubation, several cardiorespiratory variability parameters were associated with successful extubation with moderate predictive accuracy.
Impact:
-
Measures of cardiorespiratory variability, performed in extremely preterm infants while receiving NCPAP, NIPPV, and NIV-NAVA shortly after extubation, were significantly different between patients that succeeded or failed extubation.
-
Cardiorespiratory variability measures had a moderate predictive accuracy for extubation success and can be potentially used as biomarkers, in recently extubated infants.
-
Future investigations in this population may also consider including cardiorespiratory variability measures when assessing types of post-extubation respiratory support and promote individualized care.
Similar content being viewed by others
Introduction
Extubation readiness in extremely preterm infants is a complex medical issue difficult to address with simple solutions.1 Given the lack of accurate predictors, physicians often make decisions about extubation based on their clinical judgment,2 which might partially explain their high failure rates.3 After extubation, the use of non-invasive respiratory support decreases the risk of extubation failure.4 Most common modes used are nasal continuous positive airway pressure (NCPAP) and non-synchronized nasal intermittent positive pressure ventilation (ns-NIPPV). The best choice remains controversial as meta-analyses of several studies showed very low precision and high degree of bias, and the largest RCT was not able to demonstrate any superiority between CPAP or NIPPV.5,6 Synchronization during NIPPV has also been postulated as an important requirement for successful outcomes.7,8 Non-invasive neurally adjusted ventilatory assist (NIV-NAVA) can provide ventilation that is proportional and synchronized to the patient’s respiratory drive using the electrical activity of the diaphragm. Synchronized NIPPV has showed encouraging preliminary findings for reduction of extubation failure rates.9,10,11
Heart rate and respiratory rate are typical vital signs measured during respiratory support, but interpretation of those measurements is often limited to trends or averages over time. More sophisticated analyses of those signals could provide additional information on the individual’s clinical status, physiological response to treatment, and even predict outcomes.12,13,14,15,16,17,18,19,20,21,22 In fact, measurements of heart rate variability (HRV) have been able to identify system instabilities before any clinical signs of deterioration, as demonstrated for the prediction of sepsis12 or outcomes of hypoxic-ischemic encephalopathy.13 More importantly, various cardiorespiratory measures have also been found to be predictive of extubation outcomes in mechanically ventilated preterm infants, including HRV,14,15,16,17,18,19 diaphragmatic activity,20,21 and respiratory variability (RV).21,22
Given that mechanical ventilation is associated with high rates of morbidities, physicians commonly decide towards early disconnection despite the dilemma around extubation readiness. Thus, early identification of infants at increased risk of failure shortly after extubation may also be helpful to identify higher risks patients and maybe guide clinical care. In this study we aim to investigate the association between cardiorespiratory measures performed shortly after extubation and extubation outcomes in infants receiving three common modes of non-invasive respiratory support: NIV-NAVA, NCPAP and NIPPV. Furthermore, we also assessed the predictive ability of those measures to identify successful extubation.
Methods
Population
This is a secondary analysis of a prospective, unblinded, randomized crossover trial from a level III neonatal intensive care unit in Montreal, Canada (ClinicalTrials.gov Identifier: NCT02723123). Between July 2016 and August 2018, the study enrolled infants with birth weight (BW) ≤ 1250 g who were electively extubated for the first time from mechanical ventilation (MV) to non-invasive respiratory support. Infants with neuromuscular disease, major congenital anomalies, diaphragmatic paralysis or palsy, diagnosed phrenic nerve injury, hemodynamic instability, esophageal perforation, congenital heart defects, or receiving inotropes, narcotics, or sedative agents were excluded. All clinical decisions related to pre- and post-extubation ventilatory parameters, extubation, type of post-extubation respiratory support provided outside of the study period, and reintubation were made by the medical team but data was recorded and reported here. As part of institutional practices, all extremely preterm infants were extubated to either NCPAP or non-synchronized NIPPV outside of the study period, and oxygen was titrated to maintain oxygen saturation targets between 88–92%. The unit has guidelines defining non-invasive support failure, and therefore, need of reintubation. Those criteria include: (a) fraction of inspired oxygen (FiO2) consistently > 50% for 1 to 2 h, (b) severe respiratory acidosis: PCO2 > 65 mmHg with a pH < 7.20 in 2 consecutive arterial/venous blood gases, (c) two episodes of apnea requiring bag/mask ventilation due to failure to resume spontaneous breathing, (d) frequent episodes of apnea and/or bradycardia (>6 episodes in 6 h), and (e) significant increase in work of breathing. However, the medical team had the final decision concerning reintubation. Thus, the primary reason for reintubation provided by the medical team was also collected. The study was approved by the institutional research ethics board of the McGill University Health Center and informed consent was obtained from parents or legal guardians.
Study design and data acquisition
Electrocardiogram (ECG) and the electrical activity of the diaphragm (Edi) signals were collected during the study period. Prior to extubation, 3 additional ECG leads were placed on the infants’ limbs (at least 1 cm apart from existing leads to prevent interference) and a NAVA catheter with embedded electrodes (6-French/49 cm specialized feeding tube) was placed at the level of the diaphragm. The catheter connected to the NAVA module of the Servo-i ventilator (Maquet Critical Care, Solna, Sweden) and its correct positioning was confirmed as previously described.23 Immediately following extubation, infants received the type of non-invasive respiratory support prescribed by the medical team (NCPAP or ns-NIPPV) for 30–60 min to allow stabilization before initiating the recordings. After that, infants then received the study interventions (NCPAP, NIPPV, and NIV-NAVA) while in a resting supine position, and for 40 min on each mode. The first 10 min of recordings were a priori decided to be excluded from analysis to provide a wash-out period between modes. The interventions were provided in a random order determined by a computer-generated randomization sequence, generated by author G.S. and implemented by authors S.L., M.B., and W.S. (allocation was not concealed). A timeline of the study design is provided in Supplemental Fig. S1.
To avoid multiple changes, all modes of non-invasive support were delivered by the Servo-i ventilator (Maquet Critical Care, Solna, Sweden) using infant binasal prongs (Hudson RCI, Wayne, Pennsylvania). The positive end-expiratory pressures (PEEP) or NCPAP levels matched the post-extubation stabilization period pressures prescribed by the attending physician. The pressure control mode on the Servo-i ventilator was used to provide NIPPV, with peak inflation pressures (PIP) set to 10 cmH2O above the PEEP at a rate of 20 breaths per minute. If deemed necessary by the research staff or medical team for infant stability, increases in PIP and rates were permitted, with any such deviations recorded. For NIV-NAVA, the NAVA levels were adjusted in attempt to match the PIP levels provided during NIPPV. The pressure waveforms from the Servo-i were analyzed offline to determine the number of PIPs delivered, median PIP value, and MAP during NIPPV and NIV-NAVA. The occurrence of bradycardias (heart rate <100 bpm) and desaturations (SpO2 < 85%) detected by the hospital’s monitoring system were also manually recorded by the research team at the bedside (excluding those during wash-out periods), as these are the events that the medical team responds with any intervention. Changes in the FiO2 were also recorded.
Heart rate variability
Using a portable data acquisition system (FE132 Bio Amp and PowerLab, ADInstruments, Colorado, U.S.A), ECG signals were amplified, anti-alias filtered, sampled at 1000 Hz, and stored in a research computer for offline analysis using the LabChart software (Version 8.1.4, ADInstruments). ECG signals were analyzed using an in-house developed program, with documentation of the algorithm and MATLAB (MathWorks, Massachusetts, U.S.A.) application being publicly available at http://hrvtoolkit.com. Analyses were performed using the full 30-min segment.
For HRV, the following time domain, frequency domain, and non-linear parameters were calculated:24,25
Time domain
Minimum, maximum, and median NN (normal-to-normal, or R-wave-to-R-wave) interval; standard deviation of the NN intervals, i.e., the variation of intervals measured between consecutive sinus beats (SDNN); the standard deviation of the averages of NN intervals in all 5-min segments of the recording (SDANN); the mean of the standard deviations of all NN intervals for all 5-mine segments of the recording (SDNNi); the percentage of adjacent NN intervals differing by >50 ms (pNN50); root mean square of successive differences of NN intervals (RMSSD); total number of all NN intervals divided by the height of the 32-bin histogram of all NN intervals (triangular index; HRVTi); and the baseline width of the minimum square difference triangular interpolation of the highest peak of the 32-bin histogram of all NN intervals (TINN).25
Frequency domain
TP: total power (<0.4 Hz), VLF: very low frequency (<0.04 Hz), LF: low frequency (0.04–0.15 Hz), HF: high frequency (>0.15 to <0.4 Hz), and LF/HF ratio using the Welch periodogram.25
Non-linear
Sample entropy (SampEn; r = 0.1, m = 3), a measure of chaos or disorder in the NN series; detrended fluctuations analysis (DFA) scaling exponents alpha1 (short-term; 4–13 beats), and alpha2 (long-term; 14–100 beats), measures of self-similarity, or repeated patterns when inspected on different scales within the NN series; the standard deviation of intervals perpendicular to the identity line of the Poincaré plot (SD1), a measure of short-term variability, and the standard deviation of intervals along the identity line (SD2), a measure of overall variability.24
Respiratory analysis
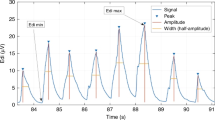
Edi signals were acquired at a sampling frequency of 100 Hz and stored for offline analysis using the Servo Tracker software (Maquet Critical Care, Solna, Sweden). For each patient and mode, the full 30-min segment of Edi signals were analyzed using an automated algorithm developed on MATLAB (R2017a, MathWorks, Natick, U.S.A.), with peak detection functions using minimum amplitude of 1 μV and at least 0.5 s apart. An example of the Edi analysis is provided in Supplemental Fig. S2.21 The median values of the following parameters were calculated and used for comparisons:
Diaphragmatic activity
The entire Edi signal was used to calculate: (a) minimum value of Edi (Edi min), i.e., the resting tonic state; (b) maximum value of Edi (Edi max), i.e., the maximal inspiratory effort; and (c) area under the entire signal (Edi area), i.e., an overall assessment of respiratory effort. A breath-by-breath analysis was used to calculate: (a) breath area, i.e., area under the breath curve; (b) breath amplitude, i.e., the minimum vertical distance that the signal descends on either side of the peak (peak-to-trough distance); (c) breath width, i.e., the distance between the points to the left and right of the peak at half the amplitude; (d) neural inspiratory time (NTi), i.e., the time from trough to peak; and (e) neural expiratory time (NTe), i.e., the time from peak to trough.
Respiratory variability
Breath-to-breath (BB) time intervals were obtained and the following measures of variability were computed for breath areas, NTi, NTe, breath amplitudes, and widths (obtained through the diaphragmatic activity breath-by-breath analysis described above): standard deviation (SD), coefficient of variation (CV), and the standard deviation of the successive differences (SDSD).21
Clinical data
The following baseline patient demographics were prospectively collected: BW, gestational age, sex, APGAR score at 5 min, delivery room intubation, age at intubation, surfactant use, weight at extubation, postnatal age and post-menstrual age at extubation, days on MV, pre-extubation blood gas (pH, partial pressure of CO2, bicarbonate, base excess) and ventilator settings (ventilation mode, PIP, PEEP, mean airway pressure (MAP), FiO2, and inflation rate), and non-invasive respiratory support provided after the study (mode, PIP, PEEP, and inflation rates). Extubation failure was defined as reintubation within 7 days of extubation, as this seems to be an optimal timeframe to capture as many respiratory-related reasons for failure while excluding most non-respiratory-related failures.26
Sample size and statistical analysis
For the crossover trial, a convenience sample size of 30 infants was chosen, with an expected loss of patients up to 20–25% for poor signal quality, technical issues, clinical instability, or need of immediate reintubation. (4,5) Given that this was a post hoc analysis, there was no pre-determined sample size based on extubation outcomes. All cardiorespiratory and clinical variables are expressed as median [IQR] or number (%) and were compared using the Wilcoxon rank sum test, or the Chi-square or Fisher’s exact tests. Given the primary results of this crossover trial demonstrating significant differences in cardiorespiratory variability measures between the modes of support,27 success and failure groups were compared while on each mode separately. Statistics were computed using MATLAB (R2018b, MathWorks, Massachusetts, U.S.A.) and a p-value < 0.05 was considered significant. Significant parameters were further evaluated for their ability to identify extubation success by calculating the area under the receiver-operator characteristics curves (AUCROC), sensitivity and specificity.
Results
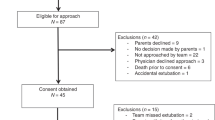
A total of 30 infants were studied and 23 (77%) had full data recordings for analysis; a flow diagram of patient recruitment is provided in Supplemental Fig. S3. Fourteen infants were successfully extubated while 9 failed, yielding a 39% reintubation rate. All failure infants were reintubated for the primary reason of apneas and bradycardias according to the medical clinical team. The median time at reintubation was 1.6 days (37 h) after extubation, with a range from 0.6 to 4.8 days (13 to 116 h). In this high-risk group of infants for extubation failure, there were no significant differences between success and failure for any pre-extubation clinical variable. Patient demographics are presented in Table 1.
All settings during the study period for each mode are provided in Table 2. Some individualized setting changes were made in 2 infants based on clinical judgment: (1) PIP/PEEP changed to 20/8 and rate of 40 ipm during NIPPV, and (2) rate increased to 40 ipm during NIPPV. There were no significant differences in the minimum or maximum FiO2 provided, or presence of desaturation or bradycardia events between the three modes (Table 2).
Heart rate variability
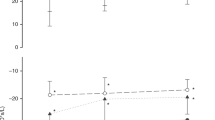
Compared to infants successfully extubated, VLF power was significantly higher in the failure group during NIV-NAVA (174.6 [108.6–1252.4] vs. 70.6 [48.9–269.2] ms2, p = 0.041; Fig. 1). Also, failure infants had significantly lower SampEn during NCPAP (0.8 [0.6–0.9] vs. 1.5 [0.8–1.7], p = 0.030; Fig. 1) and NIPPV (0.7 [0.6–0.9] vs. 1.1 [0.9–1.6], p = 0.030) when compared to success infants, with no differences during NIV-NAVA mode. Complete HRV results are provided as Supplemental Table S1. Both variables had a moderate predictive value for early detection of extubation failure, with an area under the receiver-operator characteristics (AUCROC) of 0.76 for VLF power during NIV-NAVA, and 0.78 for SampEn during both NCPAP and NIPPV (Table 3). Sensitivities for these two HRV parameters ranged from 64.3% to 85.7%, and specificities from 66.7% to 88.9% (Table 3).
Extubation success group is represented in blue (left box plot) and failures in red (right box plot) for each mode. VLF very low frequency, NCPAP nasal continuous positive airway pressure, NIPPV nasal intermittent positive pressure ventilation, NIV-NAVA non-invasive neurally adjusted ventilatory assist, red cross = outliers, *success vs. failure p < 0.05 by Wilcoxon Rank Sum test.
Diaphragmatic activity and respiratory variability
There were no significant differences in diaphragmatic activity noted between success and failure infants, or for subgroups of success and failure infants between each mode. Complete diaphragmatic activity results are provided as Supplemental Table S2.
Infants who failed extubation had significantly higher respiratory variability in breath areas, breath amplitudes, and NTe (Fig. 2). For the breath areas, failure infants had higher SDArea during NCPAP (372 [343–504] vs. 232 [141–409] uV•s, p = 0.047) and NIV-NAVA (454 [394–634] vs. 258 [157–462] uV•s, p = 0.041) and SDSDArea during NCPAP (505 vs. 294 uV•s, p = 0.047). For the breath amplitudes, failure infants had higher SDAmp during NCPAP (8.09 [6.35–8.55] vs. 4.74 [3.00–7.32] μV, p = 0.035) and NIV-NAVA (7.69 [7.54–8.41] vs. 4.37 [3.15–6.64] μV, p = 0.018), and SDSDAmp (10.28 [9.07–11.94] vs. 5.83 [3.95–8.47] μV, p = 0.047) during NIV-NAVA. For the NTe, failure infants had significantly higher CVNTe (0.71 [0.61–0.88] vs. 0.51 [0.48–0.67], p = 0.047). Complete respiratory variability results are provided as Supplemental Table S3. Respiratory variability parameters also had AUCROC values ranging from 0.75 to 0.80, with sensitivities from 64.3% to 78.6% and specificities from 77.8% to 100% (Table 3).
Extubation success group is represented in blue (left box plot) and failures in red (right box plot) for each mode. SD standard deviation, CV coefficient of variation; SDSD standard deviation of successive differences, NTe neural expiratory time, NCPAP nasal continuous positive airway pressure, NIPPV nasal intermittent positive pressure ventilation, NIV-NAVA non-invasive neurally adjusted ventilatory assist, *success vs. failure p < 0.05 by Wilcoxon Rank Sum test.
Discussion
In this secondary analysis of a randomized crossover trial, infants who subsequently succeeded or failed their extubation attempt demonstrated significant differences in cardiorespiratory variability (HRV and RV) shortly after extubation while receiving NCPAP, NIPPV, and NIV-NAVA in a random order. Although the significant differences were observed during certain modes of respiratory support, similar trends were noted during all modalities, with higher RV and VLF power (HRV), and lower sample entropy (HRV) observed in infants that failed their extubation, with a moderate accuracy in predicting successful extubation. Interestingly, different physiological responses to the tested modes of non-invasive respiratory support between those who fail vs. succeed extubation were noted even though no differences were observed in clinical stability or oxygen requirements during the recordings.
Heart rate variability
HRV is being increasingly explored as an extubation outcome predictor in preterm infants, with significant differences found both pre- and post-extubation.14,15,16,17,18,19 During the post-extubation period, heart rate characteristics, a composite measure including SDRR and SampEn, was found to be increased (indicating a reduction in HRV) in patients that went on to fail their extubation attempt.15 Furthermore, similar studies conducted by our research group have found different responses between successful and failed extubation attempts during different modes of non-invasive respiratory support.18,19 Indeed, in the present study, we found a significant increase in VLF power during NIV-NAVA, with similar trends observed during the other modes, and significant reductions in SampEn during NCPAP and NIPPV in the failure group. While the physiological significance of VLF power is not well defined and its interpretation is limited,25 studies in newborns have found changes in VLF with respect to thermoregulation,28 maturation,29,30 and sleep states.31 These results contradict previous findings, where sicker infants typically have reduced VLF (and other frequency ranges), as shown in newborns infected with respiratory syncytial virus32 and with increasing severity of hypoxic-ischemic encephalopathy.33 This raises the possibility that synchronization during non-invasive support was able to affect and uncover an ANS dysfunction amongst infants that went on failing. Nevertheless, given its unclear physiological significance, interpretations of VLF power are speculative and require further investigations.
In contrast, SampEn has demonstrated greater clinical utility. SampEn is a measure of regularity, chaos, and unpredictability, with a zero value indicating a perfectly regular signal and a value of 2 indicating a completely random signal. While previous studies have found changes in SampEn in neonates with positioning,34,35,36 pain,37,38 and maturational changes,35,39 it’s been most useful in the early identification of sepsis. Integrated into heart rate characteristics monitoring, reductions in SampEn have been predictive of impending sepsis in preterm infants, which has been characterized by low baseline variability with transient decelerations.40,41,42 While the physiological mechanism for this is unclear,41 this is in line with our findings of reduced SampEn in the failure group. Interestingly, both VLF and SampEn had moderate strength in predicting extubation outcomes (AUCROC 0.76–0.78) shortly after extubation.
Respiratory variability
Changes in RV, both increases and decreases, have been associated with worse respiratory and extubation outcomes.21,22,43,44 An increased RV for multiple parameters of respiratory depth and timing was observed in the failure group during NCPAP and NIV-NAVA, with a similar trend during NIPPV. Unstable breathing patterns will increase RV, as has been observed in infants with sepsis and necrotizing enterocolitis where instability was characterized by increased apneas, pauses, or periodic breathing.45,46 Indeed, RV was evaluated in extremely preterm infants receiving mechanical ventilation and a period of endotracheal CPAP prior to extubation and a significantly increased RV was also noted in the failure group during both types of support.21 It should be noted that 11/13 (85%) infants from that study were also included in the present study, making the general increase in RV of the failure group consistent from the pre- to the post-extubation period. Like HRV, RV parameters also had moderate strength in predicting extubation outcomes, with AUCROC ranging from 0.75–0.80. Thus, future studies attempting to predict extubation outcomes should consider incorporating measures of cardiorespiratory variability to identify a higher risk population likely to benefit from any intervention.
Study limitations and strengths
This study has some limitations. As a secondary analysis comparing two subgroups, the sample sizes within each group were small, increasing the risk of Type 1 and Type 2 errors, and multiple comparative analyses were performed. Given the exploratory nature of this analysis, these findings should be interpreted with caution but encourage future and larger studies, assisting in their design planning and parameter selection. Larger sample sizes may have provided greater insight into how the subgroups responded to the different modes of support. Seven patients (23%) were lost due to NCPAP failure or technical issues. The presence of desaturations and bradycardia events were manually recorded as free text, lacking specific information on the frequency and duration of these events. Exposure times were 40 min on each mode of support, and it is unclear what changes may have occurred with longer exposure times, such as clinical adaptations or deteriorations. The definition of extubation failure was reintubation within 7 days; however, a different timeframe could have potential effects on the predictive ability of the cardiorespiratory analyses. Future studies with larger sample sizes should explore the effect of shorter and longer definition timeframes. Furthermore, it is unclear if these findings are generalizable beyond the first extubation attempt to subsequent extubation attempts in the event of failure. The NIV-NAVA levels provided in this study were selected in an attempt to match the PIP delivered to the infant while on NIPPV. Interestingly, the levels needed for that were similar to the ones used during regular clinical care in our unit. This study also has some important strengths. It collected cardiorespiratory signals in a high-risk (39% failure rate) group of extremely preterm infants during the critical time period shortly after extubation. Three commonly used modes of non-invasive respiratory support were tested on each patient in a random order and a comprehensive analysis of cardiorespiratory signals were performed. The cardiorespiratory variability differences between successful and failed extubations had similarities across all three modes, indicating a potential generalizability of the findings regardless of the non-invasive respiratory support modality used; however, this requires validation in a larger study over a longer study period.
Conclusion
In a high-risk cohort of extremely preterm infants, measures of cardiorespiratory variability performed shortly after extubation were significantly different between infants who subsequently succeed or fail. Indeed, HRV and RV had moderate predictive accuracy for extubation success, demonstrating potential to be used as biomarkers for early identification of infants at higher risk of failure. However, as a secondary, exploratory study with a small sample size, larger studies are needed to validate the findings. Future investigations using practical tools, able to yield cardiorespiratory variability results within a few minutes or hours, may improve its prognostic ability, which will be important for individually guided therapies in trials or clinical practice.
Data availability
Research data are not shared.
References
Shalish, W. & Sant’Anna, G. M. Deciphering extubation failure in extremely preterm infants: time to embrace complexity and move forward. J. Pediatrics 194, 263 (2018).
Shalish, W., Latremouille, S., Papenburg, J. & Sant’Anna, G. M. Predictors of extubation readiness in preterm infants: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 104, F89–f97 (2019).
Mehta, P., Berger, J., Bucholz, E. & Bhandari, V. Factors affecting nasal intermittent positive pressure ventilation failure and impact on bronchopulmonary dysplasia in neonates. J. Perinatol. 34, 754–760 (2014).
Ferguson, K. N., Roberts, C. T., Manley, B. J. & Davis, P. G. Interventions to improve rates of successful extubation in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 171, 165–174 (2017).
Cummings, J. J. & Polin, R. A. Noninvasive respiratory support. Pediatrics 137, https://doi.org/10.1542/peds.2015-3758 (2016).
Lemyre, B., Davis, P. G., De Paoli, A. G. & Kirpalani, H. Nasal intermittent positive pressure ventilation (Nippv) versus nasal continuous positive airway pressure (Ncpap) for preterm neonates after extubation. Cochrane Database Syst. Rev. 2, Cd003212 (2017).
Moretti, C. et al. Nasal flow-synchronized intermittent positive pressure ventilation to facilitate weaning in very low-birthweight infants: unmasked randomized controlled trial. Pediatr. Int. 50, 85–91 (2008).
Khalaf, M. N., Brodsky, N., Hurley, J. & Bhandari, V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics 108, 13–17 (2001).
Lee, B. K., Shin, S. H., Jung, Y. H., Kim, E. K. & Kim, H. S. Comparison of Niv-Nava and Ncpap in facilitating extubation for very preterm infants. BMC Pediatr. 19, 298 (2019).
Makker, K. et al. Comparison of extubation success using noninvasive positive pressure ventilation (NIPPV) versus noninvasive neurally adjusted ventilatory assist (NI-NAVA). J. Perinatol. 40, 1202–1210 (2020).
Yagui, A. C. Z. et al. Is noninvasive neurally adjusted ventilatory assistance (NIV-NAVA) an alternative to NCPAP in preventing extubation failure in preterm infants? J. Matern. Fetal Neonatal. Med. 34, 3756–3760 (2019).
Fairchild, K. D. et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr. Res. 74, 570–575 (2013).
Goulding, R. M. et al. Heart rate variability in hypoxic ischemic encephalopathy: correlation with eeg grade and 2-Y neurodevelopmental outcome. Pediatr. Res. 77, 681–687 (2015).
Chakraborty, M., Watkins, W. J., Tansey, K., King, W. E. & Banerjee, S. Predicting extubation outcomes using heart rate characteristics index in preterm infants: a cohort study. Eur. Respir. J. 22, 22 (2020).
Goel, N., Chakraborty, M., Watkins, W. J. & Banerjee, S. Predicting extubation outcomes—a model incorporating heart rate characteristics index. J. Pediatrics 195, 53–58.e51 (2018).
Kaczmarek, J. et al. Heart rate variability and extubation readiness in extremely preterm infants. Neonatology 104, 42–48 (2013).
Silva, M. G. F., Gregorio, M. L. & de Godoy, M. F. Does heart rate variability improve prediction of failed extubation in preterm infants? J. Perinat. Med 47, 252–257 (2019).
Latremouille, S. et al. Heart rate variability in extremely preterm infants receiving nasal Cpap and non-synchronized noninvasive ventilation immediately after extubation. Respir. Care 63, 62–69 (2018).
Latremouille, S. et al. The effects of nasal continuous positive airway pressure and high flow nasal cannula on heart rate variability in extremely preterm infants after extubation: a randomized crossover trial. Pediatr. Pulmonol. 54, 788–796 (2019).
Iyer, N. P. et al. Neural breathing pattern in newborn infants pre‐and postextubation. Acta Paediatr. 106, 1928–1933 (2017).
Latremouille, S., Bhuller, M., Rao, S., Shalish, W. & Sant’Anna, G. Diaphragmatic activity and neural breathing variability during a 5-min endotracheal continuous positive airway pressure trial in extremely preterm infants. Pediatr. Res. 89, 1810–1817 (2020).
Kaczmarek, J., Kamlin, C. O., Morley, C. J., Davis, P. G. & Sant’anna, G. M. Variability of respiratory parameters and extubation readiness in ventilated neonates. Arch. Dis. Child Fetal Neonatal Ed. 98, F70–F73 (2013).
Stein, H., Hall, R., Davis, K. & White, D. B. Electrical activity of the diaphragm (Edi) values and Edi catheter placement in non-ventilated preterm neonates. J. Perinatol. 33, 707–711 (2013).
Sassi, R. et al. Advances in heart rate variability signal analysis: Joint Position Statement by the E-Cardiology Esc Working Group and the European Heart Rhythm Association Co-Endorsed by the Asia Pacific Heart Rhythm Society. Europace 17, 1341–1353 (2015).
Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart Rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065 (1996).
Shalish, W. et al. Patterns of reintubation in extremely preterm infants: a longitudinal cohort study. Pediatr. Res. 83, 969–975 (2018).
Latremouille, S., Bhuller, M., Shalish, W. & Sant’Anna, G. Cardiorespiratory effects of NIV-NAVA, NIPPV, and NCPAP shortly after extubation in extremely preterm infants: a randomized crossover trial. Pediatr. Pulmonol. 56, 3273–3282 (2021).
Stephan-Blanchard, E. et al. Heart rate variability in sleeping preterm neonates exposed to cool and warm thermal conditions. PLoS ONE [Electron. Resour.] 8, e68211 (2013).
De Rogalski Landrot, I. et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Autonomic Neurosci.-Basic Clin. 136, 105–109 (2007).
Patural, H. et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum. Dev. 84, 681–687 (2008).
Stephan-Blanchard, E. et al. The dynamics of cardiac autonomic control in sleeping preterm neonates exposed in utero to smoking. Clin. Neurophysiol. 127, 2871–2877 (2016).
Stock, C. et al. Autonomic dysfunction with early respiratory syncytial virus-related infection. Auton. Neurosci. 156, 90–95 (2010).
Goulding, R. M. et al. Heart rate variability in hypoxic ischemic encephalopathy during therapeutic hypothermia. Pediatr. Res. 81, 609–615 (2017).
Lucchini, M., Signorini, M. G., Fifer, W. P. & Sahni, R. Multi-parametric heart rate analysis in premature babies exposed to sudden infant death syndrome. Conf. Proc.: Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 6389–6392 (2014).
Lucchini, M., Fifer, W. P., Sahni, R. & Signorini, M. G. Novel heart rate parameters for the assessment of autonomic nervous system function in premature infants. Physiol. Meas. 37, 1436–1446 (2016).
Gomes, E. et al. Autonomic responses of premature newborns to body position and environmental noise in the neonatal intensive care unit. Rev. Brasileira de. Ter. Intensiv. 31, 296–302 (2019).
Weissman, A., Zimmer, E. Z., Aranovitch, M. & Blazer, S. Heart rate dynamics during acute pain in newborns. Pflügers Arch.-Eur. J. Physiol. 464, 593–599 (2012).
Cremillieux, C., Makhlouf, A., Pichot, V., Trombert, B. & Patural, H. Objective assessment of induced acute pain in neonatology with the newborn infant parasympathetic evaluation index. Eur. J. Pain. 22, 1071–1079 (2018).
de Souza Filho, L. F. M. et al. Evaluation of the autonomic nervous system by analysis of heart rate variability in the preterm infants. BMC Cardiovasc. Disord. 19, 198 (2019).
Beuchee, A. et al. Uncorrelated randomness of the heart rate is associated with sepsis in sick premature infants. Neonatology 96, 109–114 (2009).
Lake, D. E., Richman, J. S., Griffin, M. P. & Moorman, J. R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regulatory Integr. Comp. Physiol. 283, R789–R797 (2002).
Griffin, M. P. et al. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr. Res. 53, 920–926 (2003).
Hutten, G. J. et al. Respiratory muscle activity related to flow and lung volume in preterm infants compared with term infants. Pediatr. Res. 68, 339 (2010).
Usemann, J. et al. Variability of tidal breathing parameters in preterm infants and associations with respiratory morbidity during infancy: a cohort study. J. Pediatr. 205, e61 (2019).
Navarro, X., Porée, F., Beuchée, A. & Carrault, G. Artifact rejection and cycle detection in immature breathing: application to the early detection of neonatal sepsis. Biomed. Signal Process Control 16, 9–16 (2015).
Patel, M. et al. Clinical associations with immature breathing in preterm infants: part 2—periodic breathing. Pediatr. Res. 80, 28–34 (2016).
Funding
This work was supported by the Montreal Children’s Foundation research funds for G.S. In addition, S.L. and W.S. were both supported from Fonds de la recherche en sante du Quebec (FRQS) doctoral research awards in partnership with the Fondation Des Étoiles.
Author information
Authors and Affiliations
Contributions
S.L. assisted in patient screening and enrollment, was responsible for data acquisition, analyzed the data, and drafted the manuscript. M.B. assisted in patient screening and enrollment, and data acquisition. W.S. assisted in patient screening, enrollment, and data acquisition, and provided critical input into study design, data analyses, and writing of the manuscript. G.S. supervised the design and execution of the study, and provided critical input into the final data analyses and writing of the manuscript. All authors critically reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed consent was obtained from parents or legal guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Latremouille, S., Bhuller, M., Shalish, W. et al. Cardiorespiratory measures shortly after extubation and extubation outcomes in extremely preterm infants. Pediatr Res 93, 1687–1693 (2023). https://doi.org/10.1038/s41390-022-02284-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02284-5
This article is cited by
-
Peri-extubation settings in preterm neonates: a systematic review and meta-analysis
Journal of Perinatology (2024)