Abstract
Background
Maintaining of remission early in the disease course of Crohn’s disease (CD) is essential and has major impact on the future prognosis. This study aimed to identify baseline predictors to develop model allowing stratification of patients who will not benefit from long-term azathioprine (AZA) treatment and will require more intensive therapy.
Methods
This study was designed to develop clinical prediction rule using retrospective data analysis of pediatric CD patients included in prospective inception cohort. Clinical relapse was defined as necessity of re-induction of remission. Sequence of Cox models was fitted to predict risk of relapse.
Results
Out of 1190 CD patients from 13 European centers, 441 were included, 50.3% patients did not experience clinical relapse within 2 years of AZA treatment initiation. Median time to relapse was 2.11 (CI 1.59–2.46) years. Of all the tested parameters available at diagnosis, six were significant in multivariate analyses: C-reactive protein (p = 0.038), body mass index Z-score >0.8 SD (p = 0.002), abnormal sigmoid imaging (p = 0.039), abnormal esophageal endoscopy (p = 0.005), ileocolonic localization (p = 0.023), AZA dose in specific age category (p = 0.031).
Conclusions
Although the possibility of predicting relapse on AZA treatment appears limited, we developed predictive model based on six baseline parameters potentially helpful in clinical decision.
Impact
-
The possibility of predicting relapse on AZA treatment appears to be possible but limited.
-
We identified six independent predictors available at diagnosis of early AZA/6-MP treatment failure in pediatric CD patients.
-
Using combination of these factors, a model applicable to clinical practice was created.
-
A web-based tool, allowing estimation of individual relapse risk in pediatric CD patients on a particular therapeutic regimen, has been developed.
Similar content being viewed by others
Introduction
In most newly diagnosed pediatric patients with Crohn’s disease (CD), during or shortly after remission induction, a choice of maintenance therapy is required.1 Although the current international recommendations2 for the treatment and diagnosis of pediatric CD indicate several prognostic factors (e.g., deep ulceration, extensive form of the disease), suggesting the need for early initiation of biological therapy, these data are not entirely based on strong data evidence. And so, this selection is currently made to some extent on the basis of experts’ opinions.3
The efficacy and safety of thiopurines has been demonstrated in a randomized controlled trial, where over 90% of patients treated with 6-mercaptopurine (6-MP) retained remission after 2 years.4 Other works aimed at the same goal, but carried out in a retrospective design, did not yield such convincing results but still demonstrated a very important role of azathioprine (AZA) in the treatment of pediatric CD.5,6 Thiopurines thus remain the method of choice for maintaining remission in most pediatric patients with CD.2 In the majority of cases, the initiation of biological therapy is indicated following its failure. However, the most recent data7 suggest that the best choice might be to start treatment with anti-tumor necrosis factor-α (aTNF) drugs in pediatric patients with moderate-to-severe CD. Nevertheless, this top–down approach has its limitations, such as costs and limited duration of action of aTNF,8,9 making it challenging to apply in daily practice. Moreover, there are patients who do not require aTNF on disease onset and for whom thiopurines are a good option to maintain remission.
Many reasons pointing to the importance of choosing an appropriate therapeutic approach for pediatric patients with CD can be identified. The long-term prognosis of CD is significantly influenced by the progression of inflammation or development of complications.10 Early initiation of an intensive therapeutic regimen, including administration of aTNF drugs, could prevent the progression of inflammation and development of complications if applied correctly.11,12 Moreover, the use of monotherapy with aTNF exposes patients to a lower risk of side effects than AZA treatment.13,14 Conversely, economic constraints and limited duration of action of the biological treatment8,9 along with an increased risk of opportunistic infections and frequent need for combination therapy with immunomodulators,15,16,17,18 are the principal disadvantages of aTNF therapy. This emphasizes the need for careful selection of patients suitable for AZA treatment or early initiation of aTNF. However, there are yet no available firm criteria to clearly define this subgroup of patients.
This study primarily aimed to identify baseline predictors of AZA/6-MP treatment failure and to develop a predictive model that allows the identification of patients who are unlikely to benefit from long-term AZA/6-MP treatment and who require an intensive therapeutic approach from the time of diagnosis. The secondary goal was to assess the time to relapse on AZA/6-MP treatment. As an ancillary analysis, we intended to briefly describe the side effects associated with this treatment using a suitable subgroup of patients primarily eligible for this study.
Methods
The study was designed as a multicenter retrospective analysis of prospectively collected data in the inflammatory bowel disease (IBD) incidence registry EUROKIDS19 to develop a clinical prediction rule. The TRIPOD guidelines for the development of clinical prediction rules (https://www.tripod-statement.org/wp-content/uploads/2020/01/Tripod-Checlist-Prediction-Model-Development.pdf) were used to complete the analysis.
Subjects
This multicenter study was conducted in cooperation with European centers specialized in the management and treatment of pediatric patients diagnosed with IBD. Finally, data from 13 centers were included in the analysis.
Based on the inclusion criteria, newly diagnosed patients with CD were selected from IBD patients included in the EUROKIDS registry19 until 2017. The initial population was thus derivate from the EUROKIDS registry. Subjects were then screened in each center separately and enrolled in this study. In a first step, patients with diagnosis of CD were selected, then those who have received AZA and who met the other inclusion criteria were included. The inclusion criteria were as follows: diagnosis of CD up to 18 years of age based on the Porto criteria or revised Porto criteria,20 available data from the EUROKIDS registry, the need for an induction regimen by exclusive enteral nutrition (EEN) or corticosteroids (CS) at the time of diagnosis (including patients who needed a switch between EEN and CS during the induction phase), initiation of AZA or 6-MP treatment within 3 weeks from the time of diagnosis, complete cessation of induction treatment within 12 weeks from diagnosis and subsequent AZA/6-MP therapy at full maintenance dose (empirically or based on thiopurine methyltransferase [TPMT] status), and remission achieved at week 12 and at least 12 months of follow-up since diagnosis, unless a clinical relapse has previously occurred, i.e., an endpoint has been reached. Concomitant 5-aminosalycylates’ therapy was allowed. Finally, patients who met the exclusion criteria were excluded: patients who used a top–down strategy (e.g., induction treatment by aTNF) with active perianal disease, intra-abdominal abscess, or fistula, and with missing data on outcome. Reported side effects that required AZA/6-MP treatment discontinuation were also considered an exclusion criterion, and patients with such a history were not included in the primary analysis.
To estimate the frequency of side effects, we used a subgroup of eligible patients in whom side effects of AZA/6-MP could be assessed. The patients were selected from the entire dataset of pediatric patients diagnosed with CD. In the first place, all patients already included in the primary analysis were included in the ancillary analysis. This set was further supplemented with data from subjects who were excluded from the primary analysis, but information on possible adverse effects was clearly available for these individuals.
Measurements
The main part of the data was obtained from the international prospective incidence registry EUROKIDS, the largest European pediatric IBD database that contains detailed data on disease phenotype and patients themselves at the time of diagnosis, date of diagnosis, center, age, sex, nationality, ethnicity, body weight, body height, body mass index (BMI), endoscopic and histological findings (described separately for each bowel segment), findings on magnetic resonance enterography or other imaging methods, extraintestinal manifestations, other CD complications, and any concomitant disease. Some of the variables (age at diagnosis, BMI Z-score) were calculated as both numerical values and factors using calculated cut-offs. To detect changes in therapeutic processes in pediatric CD over time, the years of diagnosis were also classified into the following time periods: 2004–2008, 2009–2011, 2012–2014, and 2015–2017. From all the listed parameters, we selected only the following: BMI Z-score and factorized BMI Z-score at diagnosis, height Z-score at diagnosis, age at the time of diagnosis, years of diagnosis (categorized as 2004–2008, 2009–2011, 2012–2014, and 2015–2017), sex, ethnicity, family history of IBD, extraintestinal manifestation at the time of diagnosis, location of CD at diagnosis, any concomitant disease at diagnosis, findings on small bowel through magnetic resonance imaging, abdominal computer tomography, abdominal ultrasound, and capsule endoscopy examinations at diagnosis, to be tested on potential predictive value in the context of early AZA/6-MP treatment failure.
Additional parameters that seemed clinically important and were readily accessible but were not recorded in the EUROKIDS registry were collected retrospectively. The selection was partly based on previously published data5,6,21,22—serum levels of albumin, hemoglobin, C-reactive protein (CRP), blood counts of leukocytes and platelets, fecal calprotectin, TPMT status (if available), and anti-Saccharomyces cerevisiae antibodies (ASCA) immunoglobulin A (IgA) and IgG levels—all at the time of diagnosis, final maintenance dose of AZA/6-MP (milligram per kilo [mg/kg]) between the 10th and 14th week, and date of endpoint, along with the reason for treatment change (relapse, complication, drug side effect, type of side effect), or the date of maximum follow-up in cases where the endpoint did not occur.
Definition of remission, relapse, and side effect (clinical evaluation of patients)
The primary outcome of this study was the identification of predictors of AZA/6-MP treatment failure in pediatric patients with CD and creation of a predictive model enabling the differentiation of these patients at the time of diagnosis. For this purpose, the time to relapse, defined as the need for reinduction of remission (initiation of antibiotics, EEN, special diets, CS, aTNF, other biologicals [e.g., vedolizumab, ustekinumab], thalidomide or surgery [resection or stoma]) or development of CD-related complications (abscess, fistula, clinically significant stricture) or death, was monitored and considered as the endpoint. Assessment of remission was based on the decision of the treating physician, considering the clinical condition and laboratory parameters. A minimum follow-up was set to 12 months, with exception of patients who reach defined endpoint during these 12 months.
The side effects were assessed by the treating physician and categorized as nausea/vomiting, acute pancreatitis, allergic reactions, liver test abnormalities, and bone marrow suppression.
Statistical analysis
The univariate risk analysis was performed on each potential predictor by comparing empirical hazard estimates between subgroups. For continuous predictors, the subgroups were defined by empirical quartiles. Next, hazard differences between subgroups were tested using the log-rank test. In the multivariate analysis, Cox models were used to evaluate the effect of the predictors on the risk of relapse. The predictors were assessed by performing partial likelihood ratio tests comparing a model and a submodel, with p value <0.05 interpreted as a potentially significant effect. The center was always included in the model as a categorical variable. The initial model included demographic variables, such as sex, age at diagnosis, and calendar year of diagnosis. For the latter two variables, optimal transformations were identified. Next, variables appearing significant on univariate risk analyses were added to the model in the presence of other predictors and tested for significance. Interactions among the significant predictors were also added to the model and tested for significance. The final model was built on 380 patients with complete data for all the predictors, of whom 252 had a relapse observed and the rest were censored. Among the subjects that were excluded due to missing data, 34 had missing CRP measurement, 26 had missing BMI, and 17 had missing AZA dose. Some of them had multiple missing values in these three variables.
Results
Patient characteristics
At the time of data retrieval, the EUROKIDS registry included 2241 patients from participating centers with a diagnosis of IBD; after excluding all patients with a non-CD diagnosis, 1190 patients with CD were considered for the study. Among 1190 patients, 441 (251 males [56.9%], median age at diagnosis 13.67 [Inter quartile range (IQR) 11.33–15.25] years) fulfilled the inclusion criteria and did not meet the exclusion criteria. The eligibility of patients is shown in Fig. 1. Patients’ demographic and clinical characteristics according to the Paris classification are listed in Table 1. Median time of follow-up was 1.47 (IQR 0.76–3.39) years. The representation of patients in each center was as follows: 84, 18, 46, 23, 14, 6, 48, 63, 54, 33, 7, 45, and 0 subjects.
Time to relapse on AZA/6-MP treatment
The median time to clinical relapse was 2.11 (95% confidence interval [CI] 1.59–2.46) years. Remission was observed in 50.3% of patients after 2 years of AZA/6MP treatment (Fig. 2).
Univariate risk assessment
Univariate analysis was performed on data from 441 patients. Of all the tested parameters available at the time of diagnosis (Appendix Fig. S1), only the following were found significantly associated with early relapse on AZA/6-MP treatment when tested separately: CRP (p = 0.0480), Z-score of BMI (p = 0.0079) and BMI Z-score >0.8 SD (p = 0.0039), Z-score of weight (p = 0.043), age at the time of diagnosis (p = 0.0052) and at the time of onset of symptoms (p = 0.034), year of diagnosis classified into four categories (2004–2008, 2009–2011, 2012–2014, and 2015–2017) (p = 0.005), abnormal cecum imaging findings (p = 0.044), abnormal esophageal endoscopy findings (p = 0.0473), availability of determination of AZA/6-MP metabolites (p = 0.0005), TPMT genotyping accessibility (p = 0.013), and center (p = 0.0005).
In particular, the AZA/6-MP dose did not show any significant effect on relapse in the univariate analysis. The median dose administered was 2.04 mg/kg (IQR 1.77–2.27).
Multivariate risk assessment
Data from 380 patients were used for multivariate analysis. The results are presented in two parts (Table 2a, b). The first part shows the main effects of noninteracting factors: CRP at diagnosis (hazard ratio [HR] = 1.396, 95% CI 1.019–1.912, p = 0.038), BMI Z-score >0.8 SD at diagnosis (HR = 0.533, 95% CI 0.360–0.789, p = 0.002), abnormal sigmoid imaging findings at diagnosis (HR = 1.503, 95% CI 1.021–2.212, p = 0.039), abnormal esophageal endoscopy findings at diagnosis (HR = 1.712, 95% CI 1.176–2.490, p = 0.0050), and L3 classification at diagnosis (HR = 1.396, 95% CI 1.048–1.860, p = 0.023) (Table 2a). Although the center did not provide clinically relevant information, it showed a strong association with time to relapse (p = 0.0003) and thus had to remain part of the prediction model. The second part presents the interactions between AZA and age (p = 0.031). A significant protective effect was observed in the age group of 8–11 years (HR = 0.441, 95% CI 0.223–0.870, p = 0.0183), with the relapse rate reduced by half per each mg/kg increase in AZA dose (Table 2b). There were 99 (22.4%) patients in the age group 8–11 years. The centers had between 6 and 16 patients in this group, except the two smallest centers (0 and 1 subject). The proportions of patients in this age group were not significantly different between centers. The age groups varied in certain characteristics that change over time, e.g., BMI Z-score decreased with age, CRP increased with age. But in no important clinical characteristic did the age group 8–11 years differ from all the other age groups in a striking way. See the Appendix for more detailed results.
Based on the aforementioned, a web-based tool that allows the use of this prediction model in clinical practice was developed: https://gastroped.shinyapps.io/AZA_time_to_relapse/.
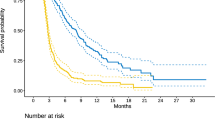
Using the final predictive model, we were able to identify two subgroups of patients who had either particularly low or particularly high risk of relapse. They were compared with hypothetically “intermediate-risk” patients. Based on the coefficients of the final model, the low-risk group was defined as follows: age at diagnosis 0–7 years with AZA dose <2 mg/kg or age 8–11 years with AZA dose >2 mg/kg or age 15–18 years with AZA dose >2 mg/kg, BMI Z-score >0.8 SD, no abnormal sigmoid imaging findings, and no abnormal endoscopic findings in the esophagus. In contrast, the high-risk group was defined as follows: age at diagnosis 12–14 years regardless of AZA dose or age 8–11 years with AZA dose <2 mg/kg, BMI Z-score <0.8, abnormal sigmoid radiology findings, or abnormal endoscopic findings in the esophagus. A comparison between the low- and high-risk groups is shown in Fig. 3.
Dark green: low-risk group (HR = 1), median time to relapse 4.98 years, relapse-free probability at 2 years 0.738. Light green: intermediate-risk group (HR = 2.59), median time to relapse 1.63 years, relapse-free probability at 2 years 0.455. Brown: high-risk group (HR = 6.54), median time to relapse 0.70 years, relapse-free probability at 2 years 0.137. Note: These results focus on clinical characteristics and do not consider between-center differences in the risk of relapse. They are all calculated for a patient located in the largest center in terms of participating patients, with medium risk among all centers. HR hazard ratio.
Side effects
Although this study was not primarily aimed at identifying the side effects of AZA/6-MP, using a group of patients for whom this information was available, we performed an ancillary analysis of this objective. A group of 1190 subjects with a diagnosis of CD considered for the primary analysis was used as the baseline data source. The follow-up subgroup in the regard of side effect consisted of all patients included in the primary analysis (n = 441) together with all patients who were previously excluded from the primary analysis based on the inclusion/exclusion criteria but for whom sufficient information on the occurrence and type of an adverse event was available (n = 101). Among all patients with data on the side effects of AZA/6-MP treatment available (n = 542), the most common side effect was bone marrow suppression (n = 31, 5.7%). Acute pancreatitis (n = 14, 2.6%), liver test abnormalities (n = 15, 2.8%), nausea/vomiting (n = 12, 2.2%), and allergic reactions (n = 1, 0.2%) were also recorded. The proportion of all patients treated with AZA/6-MP and all side effects observed in these patients is displayed in Fig. 4. AZA/6-MP treatment had to be terminated due to side effects in 8 patients (1.34%); 5 with bone marrow suppression, 1 with liver test abnormality, 1 with acute pancreatitis, 1 with lymphoma. In addition, one lymphoma developed after an outcome with a questionable relationship to the therapy, and one rhabdomyosarcoma was recorded in patients who did not stop AZA therapy before reaching the endpoint. No death was reported.
Discussion
We identified six independent predictors (BMI Z-score, age, abnormal sigmoid imaging findings, abnormal esophageal endoscopy findings, L3 classification, and CRP) available at diagnosis of early AZA/6-MP treatment failure in patients who achieved remission on EEN or CS and were concomitantly treated with AZA/6-MP. Using these data from an international multicentric registry, we developed a model for individual prediction of time to relapse in pediatric patients with CD. Although none of the predictors were strong enough to be used as a clinical tool, the model based on a combination of multiple variables could be clinically useful. However, we are aware that a prospective validation on an external cohort would be needed to evaluate the performance of the model in clinical practice. Unfortunately, we did not have enough data to conduct a serious validation of model predictions.
To the best of our knowledge, we have described the largest group of pediatric patients with CD treated with AZA/6-MP. Relapse was observed in 49.7% of patients within 2 years of AZA/6-MP treatment. This number is not as optimistic as the result published in the only randomized controlled trial in pediatric patients with IBD.4 However, it is almost equal to the findings of recent retrospective studies, which showed an almost 50% failure rate of AZA/6-MP treatment within this time period.5,6 We assume that dissimilarities in these findings may be partially caused by a common occurrence where, in general, the results of clinical studies do not achieve such success as a randomized controlled trial. The position of thiopurines in the treatment algorithm for pediatric patients with CD, respectively, IBD including ulcerative colitis, was also confirmed in a recent prospective study.23 However, a subgroup of patients who may benefit from this treatment should be defined.
Predictive factors are essential for this selection. The choice of outcome plays a very important role in the search for these predictors. For our purpose, to prevent early failure of the chosen treatment, the first relapse of the disease seems the most appropriate. Many studies describing variables predictive of general outcomes (irrespective of maintenance treatment type) in pediatric patients with CD have been published. They differ in many aspects, such as the definition of the outcome, study population, and examined variables.24,25,26,27,28 None of these studies was designed to assess the response to a specific therapeutic approach in patients with CD.
To identify those who may benefit from early initiation of biologic therapy, ECCO/ESPGHAN guidelines on the medical management of pediatric Crohn’s disease1 proposes stratification of patients based on negative prognostic criteria. In our study, we could not consider five of them. Severe perianal disease and stricture and penetrating disease; since in this case biological therapy is already indicated for initiation at the time of diagnosis, these parameters were set as exclusion criteria. Persistent severe inflammation despite adequate therapy could not be studied as a potential predictor as this was considered outcome in our work. The last two parameters that we were not able to assess were severe osteoporosis and deep ulceration, because this information was not available within our dataset. The other two parameters (growth retardation and panenteric disease at the time of diagnosis) were not shown to be predictive of early relapse on AZA treatment, although the demonstration of L3 classification as an independent predictor of early relapse could be considered as some degree of concordance with the 2014 recommendation in this regard.
Although we can use general predictors, predictors of early relapse in relation to a specific therapeutic approach are crucial. We identified only a few studies that focused on predictive factors in thiopurine monotherapy. In contrast to our study, Riello et al.6 did not identify any predictive factor in the context of early relapse defined as PCDAI >10 with no CS, need for reinduction, or change of immunomodulator. Age, sex, location and behavior of disease, perianal fistulizing disease, and extraintestinal manifestation at baseline were assessed in this retrospective study. Similar to our study, a small retrospective study primarily focusing on induction treatment modality5 found that age <16 years and involvement of the upper gastrointestinal tract at diagnosis were predictive of early relapse, defined as an increase in disease activity with the need for additional reinduction therapy in patients originally indicated for long-term immunosuppressive treatment of AZA/6-MP. We were not able to confirm other predictors identified in this study (elevated platelet count at remission and lower height Z-score at diagnosis). None of these studies described a separate predictor strong enough to be applicable in routine clinical practice. A combination of these factors may be needed for the development of clinically useful predictive tools.
A validated web-based tool displaying an individualized predicted outcome for adult patients with CD has already been developed.29 The final multivariate model in this study included disease location, serologic markers, the NOD2 frameshift mutation, and an interaction between perianal fistulizing disease and ASCA. Different outcomes (time from diagnosis to first CD-related complication regardless of therapeutic approach) and different study populations (e.g., inclusion of patients with perianal fistulizing disease, who were excluded from our dataset) have to be taken into account when considering that none of the described variables, within those included in our work as well, was found significant in our study. Waljee et al.30 focused on the prediction of remission, defined as the absence of objective evidence of intestinal inflammation and clinical outcomes in patients on thiopurines. A machine-learning algorithm was created for this purpose. The five most important variables reported in this study were hemoglobin, lymphocytes, hematocrit, neutrophils, and platelets. However, it is also difficult to compare the results from this retrospective study due to different outcomes and study populations.30 These predictive models, which do not consider a treatment approach, have certain limitations for pediatric patients, especially because they are designed for adult patients.
Even though we were not able to find a universal AZA/6-MP dose effect on relapse, the test for interaction between AZA dose and age was significant. It suggests that AZA may act differently in different age groups. Small retrospective study5 found age <16 years predictive for early relapse in patients treated with AZA/6-MP initially. On the contrary, Riello et al.6 did not find age at the time of initiation of AZA/6-MP as factors influencing the response to AZA/6-MP treatment. In this study, mean age in the relapse (11.6) and non-relapse (12.1) groups were comparable (p = 0.17). Nguyen et al.31 also showed that age does not predict efficacy of AZA treatment in pediatric CD patients (median age 13.8 (10.4–15.8) years). These results correspond to our findings that AZA/6-MP dose probably plays an important role especially in the specific age group of 8–11 years (a non-linear association due to interaction). Since we do not have any evidence explaining why the dose of AZA had an important effect in this age group, we can only speculate about slightly different pharmacokinetics of the drug in younger age groups or adolescents’ compliance to the treatment as possible explanations of this occurrence.
Within AZA dose effect assessment, we were not able to reflect AZA levels, as this information is not available in most centers. Due to the low number of patients with TPMT status tested, it was not possible to analyze whether there was any effect of lower dosing based on genotype.
The frequency of adverse events we found was consistent with their occurrence reported in other studies.32,33,34,35,36,37 Leukopenia and acute pancreatitis, the most frequently detected side effects within our dataset (5.7 and 2.6%, respectively), were reported in 10 and 4.9% of CD patients, respectively,34,37 as well as recorded thiopurine withdrawal rate of 2–30% due to side effect34,38,39 is verging to observed frequency in our study.
Although this study, performed on the largest pediatric cohort, identified useful predictors of AZA/6-MP treatment outcome, we are aware of several limitations. Validation on an external cohort could not be performed due to limited sample size and accessibility to external cohorts. Internal validation by splitting the data into a training dataset and a validation dataset could not be performed due to inability to identify any significant predictors in a smaller dataset. We believe further independent validation should be performed before the full implementation of this model into the clinical practice. As another possible limitation of the study, we perceive a possible selection bias due to the significant representation of tertiary centers and thus probably a higher proportion of complicated patients. Another limitation in this sense may be the uneven distribution of the number of patients represented from each center, which we attribute mainly to the size of the center and the established therapeutic approach. Although a multicenter study always brings positive elements, such as larger sample size or better applicability, this design bears also some disadvantages. The strong association between time to relapse and study center testified about heterogeneity of patient populations between centers and made identification of reliable predictors difficult. Even so, considering the lack of evidence to make an informed clinical decision, when choosing from available treatment options, the newly identified predictors of AZA/6-MP effect duration might help clinicians to drive the decision when managing pediatric patients with CD. For the development of the prediction tool, we used the most accurate data available. Although a part of the data was collected retrospectively, most variables were extracted from a large prospectively recorded Europe-wide database. Further studies are needed to assess whether some of the less established predictors we identified in this study will prove reliable enough for clinical practice. However, there are many factors that may influence the outcome but could not be assessed in our study, such as the effect of diet,40,41 smoking,42 or other personal habits. Therefore, the prediction of the response to a particular treatment based on the information available at the time of diagnosis is a difficult task. Since we defined relapse as the necessity of reinduction of remission, different approaches to the management and care of patients with IBD among the participating centers should be considered one of the major limitations of our study. This is another possible explanation of the strong association between center and relapse.
Although this was not the primary aim of the study, we aimed to estimate the number of side effects associated with AZA/6-MP treatment in pediatric patients with CD. However, because of the characteristics of the collected data, it is difficult to consider these numbers as relevant frequencies, as they are collected from a selected population. Moreover, due to the small number of patients who ceased therapy because of developing side effects, it was not possible to predict these particular cases.
Conclusion
In conclusion, we identified BMI Z-score, age, disease localization (abnormal sigmoid imaging findings, abnormal esophageal endoscopy findings, and L3 classification), and CRP at the time of diagnosis as independent predictive factors for early AZA/6-MP treatment failure in pediatric patients with CD. The patient’s age at diagnosis in interaction with the AZA dose may be a risk or protective factor for early relapse. However, none of these factors alone is strong enough to be used in clinical practice; therefore, using a combination of these factors, a model that might be applicable to clinical practice, and thus allows better selection of patients suitable for AZA/6-MP treatment, was created. According to our data, 50% of patients were in clinical remission after 2 years of AZA/6-MP treatment initiation; our results therefore suggest that thiopurines can still be considered a suitable treatment option for pediatric patients with CD, especially using the identified predictors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ruemmele, F. M. et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis 8, 1179–1207 (2014).
van Rheenen, P. F. et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J. Crohns Colitis 15, 171–194 (2020).
Bronsky, J. et al. Diagnostic and therapeutic approach in paediatric inflammatory bowel diseases: results from a clinical practice survey. J. Pediatr. Gastroenterol. Nutr. 68, 676–683 (2019).
Markowitz, J., Grancher, K., Kohn, N., Lesser, M. & Daum, F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology 119, 895–902 (2000).
Hradsky, O., Copova, I., Zarubova, K., Nevoral, J. & Bronsky, J. Time to relapse in children with crohn’s disease treated with azathioprine and nutritional therapy or corticosteroids. Dig. Dis. Sci. 61, 2041–2050 (2016).
Riello, L. et al. Tolerance and efficacy of azathioprine in pediatric Crohn’s disease. Inflamm. Bowel Dis. 17, 2138–2143 (2011).
Jongsma, M. M. E. et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn’s disease: an open-label multicentre randomised controlled trial. Gut 71, 34–42 (2020).
Hyams, J. et al. Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn’s disease in children: reach open-label extension. Curr. Med. Res. Opin. 27, 651–662 (2011).
Ruemmele, F. M. et al. Efficacy of infliximab in pediatric Crohn’s disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm. Bowel Dis. 15, 388–394 (2009).
Pariente, B. et al. Development of the Crohn’s disease digestive damage score, the Lemann Score. Inflamm. Bowel Dis. 17, 1415–1422 (2011).
D’Haens, G. et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 371, 660–667 (2008).
Peyrin-Biroulet, L., Bigard, M. A., Malesci, A. & Danese, S. Step-up and top-down approaches to the treatment of Crohn’s disease: early may already be too late. Gastroenterology 135, 1420–1422 (2008).
Hindorf, U., Lindqvist, M., Hildebrand, H., Fagerberg, U. & Almer, S. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment. Pharm. Ther. 24, 331–342 (2006).
Gearry, R. B., Barclay, M. L., Burt, M. J., Collett, J. A. & Chapman, B. A. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol. Drug Saf. 13, 563–567 (2004).
Bonovas, S. et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin. Gastroenterol. Hepatol. 14, 1385.e10–1397.e10 (2016).
de Bie, C. I., Escher, J. C. & de Ridder, L. Antitumor necrosis factor treatment for pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 18, 985–1002 (2012).
Subramaniam, K. et al. Hepatosplenic T-cell lymphoma, immunosuppressive agents and biologicals: what are the risks? Intern. Med. J. 44, 287–290 (2014).
Siegel, C. A., Marden, S. M., Persing, S. M., Larson, R. J. & Sands, B. E. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin. Gastroenterol. Hepatol. 7, 874–881 (2009).
de Bie, C. I. et al. Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the Eurokids Registry. Inflamm. Bowel Dis. 19, 378–385 (2013).
Levine, A. et al. ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 58, 795–806 (2014).
Jaspers, G. J. et al. Azathioprine maintains first remission in newly diagnosed pediatric Crohn’s disease. Inflamm. Bowel Dis. 12, 831–836 (2006).
Punati, J. et al. Effect of early immunomodulator use in moderate to severe pediatric Crohn disease. Inflamm. Bowel Dis. 14, 949–954 (2008).
Atia, O. et al. Role of thiopurines in pediatric inflammatory bowel diseases: a real-life prospective cohort study. J. Pediatr. Gastroenterol. Nutr. 70, 825–832 (2020).
Gupta, N. et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology 130, 1069–1077 (2006).
Henderson, P. et al. Serum C-reactive protein and CRP genotype in pediatric inflammatory bowel disease: influence on phenotype, natural history, and response to therapy. Inflamm. Bowel Dis. 21, 596–605 (2015).
Kugathasan, S. et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 389, 1710–1718 (2017).
Levine, A. et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: evaluation of the Porto IBD Group “Growth Relapse and Outcomes with Therapy” (Growth CD) Study. Inflamm. Bowel Dis. 20, 278–285 (2014).
Siegel, C. A. et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn’s disease. Inflamm. Bowel Dis. 17, 30–38 (2011).
Siegel, C. A. et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment. Pharm. Ther. 43, 262–271 (2016).
Waljee, A. K. et al. Machine learning algorithms for objective remission and clinical outcomes with thiopurines. J. Crohns Colitis 11, 801–810 (2017).
Nguyen, T. V., Vu, D. H., Nguyen, T. M., Lachaux, A. & Boulieu, R. Exploring associations of 6-thioguanine nucleotide levels and other predictive factors with therapeutic response to azathioprine in pediatric patients with ibd using multilevel analysis. Inflamm. Bowel Dis. 19, 2404–2410 (2013).
Dayharsh, G. A. et al. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 122, 72–77 (2002).
Ford, L. T. & Berg, J. D. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J. Clin. Pathol. 63, 288–295 (2010).
Kirschner, B. S. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology 115, 813–821 (1998).
Kreijne, J. E. et al. Real-life study of safety of thiopurine-allopurinol combination therapy in inflammatory bowel disease: myelotoxicity and hepatotoxicity rarely affect maintenance treatment. Aliment. Pharm. Ther. 50, 407–415 (2019).
Mottet, C. et al. Experts opinion on the practical use of azathioprine and 6-mercaptopurine in inflammatory bowel disease. Inflamm. Bowel Dis. 22, 2733–2747 (2016).
Weersma, R. K. et al. Increased incidence of azathioprine-induced pancreatitis in Crohn’s disease compared with other diseases. Aliment. Pharm. Ther. 20, 843–850 (2004).
Fuentes, D. et al. High-dose azathioprine in children with inflammatory bowel disease. Aliment. Pharm. Ther. 17, 913–921 (2003).
Papay, P. et al. The impact of thiopurines on the risk of surgical recurrence in patients with Crohn’s disease after first intestinal surgery. Am. J. Gastroenterol. 105, 1158–1164 (2010).
Levine, A. et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 157, 440.e8–450.e8 (2019).
Svolos, V. et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 156, 1354.e6–1367.e6 (2019).
Lindberg, E., Jarnerot, G. & Huitfeldt, B. Smoking in Crohn’s disease: effect on localisation and clinical course. Gut 33, 779–782 (1992).
Funding
This work was supported by the Grant Agency of Charles University in Prague [grant number 364617] and by the Ministry of Health, Czech Republic—conceptual development of research organization, Motol University Hospital, Prague, Czech Republic [00064203].
Author information
Authors and Affiliations
Contributions
T.L. worked on background research and participated in work on the conception and design of the study, data collection and its control, statistical analysis of the data, and writing of this original article. O.H. created the conception and design of the study, worked on statistical analysis of the data, revision of the original article, highlighted the potential problems and proposed the solutions, and revised the work critically for important intellectual content. M.K. worked on statistical analysis of the data and revision of the original article. G.V. participated in data collection and their control. J.A.D., M.S., S.K., S.V.B., J.M., K.W., T.d.M., and J.S. participated in data collection and their control and revision of the original article. D.E.S. and K.-L.K. participated in data collection and their control, revision of the original article, and revised the work critically for important intellectual content. J.C.E. was responsible for leading the project team, involved in study scheduling, and revised the work critically for important intellectual content. J.B. was responsible for leading the project team, involved in study scheduling, obtained the necessary documents, highlighted the potential problems and proposed the solutions, and revised the work critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
T.L. reports lectures/congress fees/consultancy (outside the submitted work): Ferring, Nutricia, Biocodex, AbbVie; O.H. reports lectures/congress fees/consultancy (outside the submitted work): MSD, AbbVie, Nutricia, Nestlé, Ferring, and Falk; M.K. declares no conflict of interest; J.A.D. reports lectures/congress fees/consultancy (outside the submitted work): Rorer, Danone, Takeda, Adacyte; M.S. declares no conflict of interest; S.K. reports lectures/congress fees/consultancy (outside the submitted work): AbbVie, Abela Pharm, Abbott Nutrition, Mead &Johnson, Nestle, Nutricia, Oktal Pharma, Shire; S.V.B. declares no conflict of interest; J.M. declares no conflict of interest; D.E.S. reports lectures/congress fees/consultancy (outside the submitted work): Abbvie, Dr. Reddy’s, Montavit, Nutricia, and Reckitt Benckiser, Noventure, Nestle, Nutricia; K.W. declares no conflict of interest; T.d.M. declares no conflict of interest; J.S. declares no conflict of interest; K.-L.K. reports lectures/congress fees/consultancy (outside the submitted work): Abbvie, Biocodex, Ferring, Tillotts PharmaFee; research Grants: Pediatric Research Foundation (Finland), Helsinki University Hospital Research Fund; J.C.E. declares no conflict of interest; J.B. reports lectures/congress fees/consultancy (outside the submitted work): MSD, AbbVie, Nutricia, Nestlé, Ferring, Biocodex, and Walmark.
Ethics approval and consent to participate
The study was approved by the ethics committee of the authors’ institution (EK-1491/16). If applicable, local ethics committee approval was obtained from the participating centers based on local requirements. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution’s human research committee. Patient informed consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lerchova, T., Hradsky, O., Kulich, M. et al. Prediction of thiopurine failure in pediatric Crohn’s disease: pediatric IBD Porto group of ESPGHAN. Pediatr Res 93, 1659–1666 (2023). https://doi.org/10.1038/s41390-022-02270-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02270-x