Abstract
Background
Preoperative urinary dickkopf-3 (DKK3) is proposed as an early biomarker for the prediction of acute kidney injury (AKI) in patients undergoing cardiac surgery. We explored the clinical utility of urinary DKK3 for the early predictive value for AKI, sepsis-associated AKI (SA-AKI), and pediatric intensive care unit (PICU) mortality in critically ill children.
Methods
Urine samples were collected during the first 24 h after admission for measurement of DKK3. AKI diagnosis was based on serum creatinine and urine output using the KDIGO criteria. SA-AKI was defined as AKI that occurred in children who met the sepsis criteria in accordance with the surviving sepsis campaign international guidelines for children.
Results
Of the 420 children, 73 developed AKI, including 24 with SA-AKI, and 30 died during the PICU stay. The urinary DKK3 level was significantly associated with AKI, SA-AKI, and PICU mortality, even after adjustment for confounders. The area under the receiver operating characteristic curve of urinary DKK3 for the discrimination of AKI, SA-AKI, and PICU mortality was 0.70, 0.80, and 0.78, respectively.
Conclusion
Urinary DKK3 was independently associated with an increased risk for AKI, SA-AKI, and PICU mortality and may be predictive of the aforementioned issues in critically ill children.
Impact
-
Urinary dickkopf-3 (DKK3) has been identified as a preoperative biomarker for the prediction of acute kidney injury (AKI) following cardiac surgery or coronary angiography in adult patients. However, little is known about the clinical utility of urinary DKK3 in pediatric cohorts.
-
This study demonstrated that urinary DKK3 is capable of early predicting AKI and pediatric intensive care unit (PICU) mortality and discriminating sepsis-associated AKI (SA-AKI) from other types of AKI.
-
Urinary DKK3 may be an early biomarker for predicting AKI, SA-AKI, and PICU mortality in critically ill children.
Similar content being viewed by others

Introduction
Acute kidney injury (AKI) is a common organ dysfunction in clinical scenarios and is associated with high morbidity and mortality in critically ill patients.1,2 One major contributing factor for the development of AKI is sepsis,3,4 which is characterized by a systemic inflammatory reaction response to infection and leads to life-threatening organic dysfunction.5 Clinically, sepsis patients complicating AKI have a 3–5-fold increase in the risk of mortality compared to sepsis patients without AKI.6 Accurate identification of patients with AKI, sepsis-associated AKI (SA-AKI), or at a high risk of mortality in the early phase of the disease could initiate effective therapeutic measures and improve patient outcomes.
Serum creatinine (sCr) and urine output are the most commonly used application tools in the measurement of renal function, but they are poor markers with insensitivity and non-specificity.7,8 An effective intervention for AKI is largely limited by the diagnosis based on changes in sCr or urine output.9,10 Within the past years, many research works have focused on identifying early biomarkers for predicting AKI.4,11 An ideal biomarker should be non-invasive and simple to detect at the early stages of the injury, and it should be specific for the affected tissue type and have a pathophysiological connection to the injury.12 However, thus far, none of the AKI biomarkers has been widely accepted and adopted in clinical practice, and strong evidence is still lacking to confirm the value of these biomarkers to predict poor prognosis.
Dickkopf-3 (DKK3) is a secreted glycoprotein belonging to the dickkopf family.13 Urinary DKK3 has been identified as a preoperative biomarker for the prediction of AKI following cardiac surgery,14 and it could be distinguished from commonly used biomarkers, such as kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), which only start to increase after kidney injury has occurred.14,15 However, DKK3 has been documented to be involved in the pathogenesis of various diseases through Wnt/β-catenin signaling pathway,16 which is associated with infection and sepsis.17,18 The results obtained from the previous study conducted in a specific setting could be applied to the general intensive care unit (ICU) population including those with sepsis remains elusive. Little is known about the discriminative ability of urinary DKK3 for sepsis and SA-AKI. In addition, the kidneys of adults are different from those of children, including immaturity of tubular function and glomerular filtration.19 Whether urinary DKK3 has a predictive performance in children is not understood yet.
The aims of this study were to investigate whether urinary DKK3 could serve as an early biomarker of AKI, to determine whether urinary DKK3 has an ability to distinguish SA-AKI from other types of AKI, and to evaluate whether urinary DKK3 is capable of predicting pediatric ICU (PICU) mortality in critically ill children.
Methods
Study population
All children admitted to the PICU in Children’s Hospital of Soochow University from December 2017 to January 2018 and September to December 2020 were considered for inclusion in this prospective study. The study exclusion criteria included age on admission <1 month or over 18 years old, the presence of a congenital renal abnormality with abnormal renal function, and a failure to collect urine samples within the first 24 h of PICU stay. The Institutional Review Board of the Children’s Hospital of Soochow University approved this study. Informed consent was obtained from each participating individual’s guardian, and all clinical investigations were conducted in accordance with the Declaration of Helsinki.
Clinical and laboratory data collection
Clinical characteristics and laboratory findings were collected on the day of admission, including age, body weight, body mass index (BMI), sex, medical history, admission diagnosis, and illness severity assessed by the Pediatric Risk of Mortality III (PRISM III) score, which is based on age-related physiological parameters recorded in the first 24 h after PICU admission.20 BMI was standardized to calculate the age, sex and length-specific standard deviation score (SDS).21Clinical status, comorbidities, medication, and therapeutic interventions were recorded daily during the PICU stay.
Definition of clinical outcomes
The diagnosis and stage of AKI were defined based on sCr and urine output using the criteria of Kidney Disease: Improving Global Outcome.22 Baseline sCr was defined as the lowest level of sCr within 3 months prior to PICU admission.2 For children whose baseline sCr was not available, we assumed a normal estimated glomerular filtration rate (eGFR) of 120 ml/min/1.73 m2 and back calculated the expected baseline sCr using the modified Schwartz estimating equation [sCr (mg/dl) = 0.413 × height (cm)/eGFR].23,24,25,26 An eGFR of 120 ml/min/1.73 m2 was selected to be consistent with previous studies of pediatric AKI.25,26 AKI stage 1 was defined as mild AKI, and AKI stages 2 and 3 were defined as severe AKI.
According to the surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children,27 critically ill children with sepsis were identified as those who had any suspected or confirmed infection accompanied by acute organ dysfunction defined by a pediatric sequential organ failure assessment score of 2 points or more,28 SA-AKI was identified as critically ill children who met the criteria for sepsis and the criteria for AKI simultaneously.
The adverse outcome of interest was PICU mortality, which was defined as all-cause mortality during the PICU stay after admission, including death resulting from the withdrawal of treatment.
Measurement of urinary DKK3
The urine samples were collected upon PICU admission and were immediately aliquoted and stored at –80 °C after collection. Every sample was first centrifuged at 3000 rpm at 4 °C for 5 min, and the supernatant was used for the measurement of urinary DKK3 and creatinine. The urinary DKK3 level was quantified by an enzyme-linked immunosorbent assay (ELISA) kit (DuoSet ELISA, DY1118, R&D Systems, Minneapolis, MN). The procedure was conducted strictly in accordance with the manufacturer’s instructions. The intra-assay and inter-assay coefficients of variation were less than 10%. The assay range was 31.2–2000 pg/ml. The DKK3 concentrations were detectable in all samples. Urinary DKK3 concentrations were corrected for urinary creatinine (urinary DKK3-to-creatinine ratio, uDKK3/Cr) to eliminate the effect of different urinary flow rates. The concentrations of urinary creatinine were measured by the sarcosine oxidase method on an automatic biochemical analyzer (Hitachi 7600, Tokyo).
Statistical analysis
Data analysis was performed using SPSS Statistical Software Version 23.0 and MedCalc Version 19.6. The assumption of normality and homogeneity of variance were checked. Continuous variables were described as median (interquartile range) and were compared using the Mann–Whitney U test or Kruskal–Wallis H test, as they were all skewed distributions. Categorical variables were described as numbers (percentage) and compared using the χ2 test or Fisher’s exact test. Spearman’s correlation analyses were performed to examine correlations between uDKK3/Cr and clinical variables. Continuous variables were log10 transformed for the generalized linear model to analyze variables potentially associated with uDKK3/Cr. Multicollinearity among variables was assessed using tolerance and variance inflation factor (VIF). Univariate and multivariate logistic regression analyses were performed and the odds ratio (OR) and adjusted OR (AOR) with a 95% confidence interval (CI) were calculated to investigate the association of uDKK3/Cr with AKI, severe AKI, sepsis, SA-AKI, and mortality. The levels of uDkk3/Cr were log10 transformed to achieve a better model fit in the logistic regression analyses. The area under the curve (AUC) of the receiver operating characteristic was calculated to assess the predictive strength, and the nonparametric method of Delong was used to compare the difference between AUCs. A two-tailed P value <0.05 was considered to be statistically significant.
Results
Patient characteristics
This prospective study involved 420 critically ill children. A total of 436 children were admitted to the PICU during the study period, and 16 were excluded: 1 was at an age <1 month, 5 had a congenital renal abnormality with abnormal renal function, and 10 had a failure in collecting urinary samples during the first 24 h after admission.
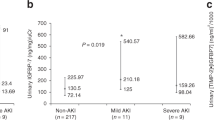
Of the 420 children, 73 developed AKI during the PICU stay, and 123 were diagnosed with sepsis. The comparisons of the demographic and clinical characteristics between non-AKI and AKI and between non-sepsis and sepsis are listed in Table 1, and the comparison of the level of uDKK3/Cr among these four groups is displayed in Fig. 1a. Moreover, of the 73 children with AKI, 35 were with AKI stage 1, 18 with stage 2, and 20 with stage 3. The comparisons of demographic and clinical characteristics grouped according to the AKI status are displayed in Supplementary Table S1. Of the 73 AKI children, 24 were diagnosed with SA-AKI, and the comparisons of demographic and clinical characteristics between children with SA-AKI and other types of AKI are displayed in Supplementary Table S2. The possible etiologies of other types of AKI are presented in Supplementary Table S3.
a With and without AKI and sepsis, b between survivors and non-survivors. Note the use of a log10 scale on the y-axis. Each circle represents an individual patient, with the horizontal lines indicating median and interquartile range. AKI acute kidney injury, uDKK3/Cr urinary dickkopf-3-to-creatinine ratio. *P < 0.05 vs. non-AKI and non-sepsis group.
The mortality rate in the whole cohort was 7.1% (95% CI: 4.7–9.6%) during the PICU stay. A comparison of the demographic and clinical characteristics between survivors and non-survivors is presented in Supplementary Table S4, and the comparison of the level of uDKK3/Cr between survivors and non-survivors is shown in Fig. 1b.
Correlation of urinary DKK3 with clinical variables
As displayed in Supplementary Fig. S1, Spearman’s correlation analyses were used to evaluate the relationship between uDKK3/Cr and variables listed in Table 1. The generalized linear model was further conducted to identify whether these variables in Table 1 were significantly associated with uDKK3/Cr after checking the multicollinearity through tolerance and VIF. As presented in Table 2, the uDKK3/Cr was independently associated with BMI SDS (P = 0.023), PRISM III score (P < 0.001), AKI stage (P = 0.030), sepsis (P = 0.004), furosemide treatment (P = 0.016), and PICU mortality (P = 0.013).
Association of urinary DKK3 with AKI and severe AKI
The comparison of the level of uDKK3/Cr grouped according to the AKI status is shown in Supplementary Fig. S2. Multiple comparisons of two groups were generated after comparison among four groups. Although the difference in the level of uDKK3/Cr between non-AKI and AKI stage 1 groups was not statistically significant (P = 0.118), the levels of uDKK3/Cr were higher in the AKI stage 2 (P = 0.002), and AKI stage 3 groups (P < 0.001) than in the non-AKI group, respectively.
To identify whether urinary DKK3 was associated with AKI and severe AKI, the univariate and multivariate logistic regression analyses were performed in Fig. 2 and Supplementary Table S5. In the univariate analysis, the uDKK3/Cr was associated with AKI (OR = 2.623, 95% CI: 1.819–3.780, P < 0.001) and severe AKI (OR = 3.589, 95% CI: 2.245–5.737, P < 0.001), and the associations of uDKK3/Cr with AKI (AOR = 2.010, 95% CI: 1.340–3.015, P = 0.001), and severe AKI (AOR = 2.284, 95% CI: 1.348–3.870, P = 0.002) remained significant after adjustment for BMI SDS, PRISM III score and the presence of sepsis in the multivariate analysis.
The uDkk3/Cr was log10 transformed to achieve a better model fit in the regression analyses. AKI acute kidney injury, AOR adjusted OR, OR odds ratio, SA-AKI sepsis-associated AKI, uDKK3/Cr urinary dickkopf-3-to-creatinine ratio. aAfter adjustment for BMI SDS, PRISM III score and sepsis. bAfter adjustment for BMI SDS, PRISM III score and AKI stage. cAfter adjustment for BMI SDS and PRISM III score. dAfter adjustment for BMI SDS, PRISM III score, AKI stage, and sepsis.
The uDKK3/Cr achieved AUCs of 0.70 (P < 0.001) and 0.76 (P < 0.001) for predicting AKI and severe AKI, which were similar to the PRISM III score for the prediction of AKI (AUC = 0.72, P < 0.001) and severe AKI (AUC = 0.77, P < 0.001), as displayed in Fig. 3a, b. However, adding the PRISM III score to a model that only included uDKK3/Cr did not improve the AUC for predicting AKI (▵AUC = 0.03, P = 0.432) or severe AKI (▵AUC = 0.02, P = 0.643).
Association of urinary DKK3 with sepsis
As shown in Fig. 2 and Supplementary Table S5, the uDKK3/Cr was associated with sepsis in the univariate analysis (OR = 2.199, 95% CI: 1.602–3.019, P < 0.001) and in the multivariate analysis after adjustment for BMI SDS, PRISM III score and AKI stage (AOR = 1.846, 95% CI: 1.299–2.622, P = 0.001). The uDKK3/Cr achieved an AUC of 0.67 (P < 0.001), which was similar to PRISM III score (AUC = 0.66, P < 0.001), for the prediction of sepsis in Fig. 3c. However, combining uDKK3/Cr with PRISM III score (AUC = 0.67, P < 0.001) did not improve the AUC for predicting sepsis (P = 0.897).
Association of urinary DKK3 with SA-AKI
Since urinary DKK3 was independently associated with AKI and sepsis, we further investigated the association between uDKK3/Cr and SA-AKI using univariate and multivariate logistic regression analyses. The uDKK3/Cr was associated with SA-AKI in the univariate analysis (OR = 4.157, 95% CI: 2.355–7.338, P < 0.001), and in the multivariate analysis after adjustment for BMI SDS and PRISM III score (AOR = 3.279, 95% CI: 1.737–6.190, P < 0.001), as shown in Fig. 2 and Supplementary Table S5. The uDKK3/Cr displayed an AUC of 0.80 (P < 0.001), which was higher than the result obtained from the PRISM III score (AUC = 0.77, P < 0.001), in predicting SA-AKI in critically ill children in Fig. 3d. However, the difference between the two AUCs did not reach statistical significance (P = 0.573). When combining uDKK3/Cr with the PRISM III score, the predictive performance (AUC = 0.80, P < 0.001) was not improved over that of uDKK3/Cr alone (P = 0.874).
Moreover, in children with sepsis (n = 123), the uDKK3/Cr was associated with AKI in the univariate analysis (OR = 3.892, 95% CI: 1.882–8.051, P < 0.001, n = 123) and in the multivariate analysis after adjustment for BMI SDS and PRISM III score (AOR = 3.370, 95% CI: 1.563–7.263, P = 0.002, n = 123). The uDKK3/Cr had an AUC of 0.75 for predicting AKI in critically ill children with sepsis. In AKI children (n = 73), the uDKK3/Cr was also significantly associated with sepsis in the univariate analysis (OR = 2.801, 95% CI: 1.294–6.064, P = 0.009, n = 73) and in the multivariate analysis after adjustment for BMI SDS and PRISM III score (AOR = 2.606, 95% CI: 1.122–6.052, P = 0.026, n = 73). The uDKK3/Cr displayed an AUC of 0.70 for predicting sepsis in critically ill children with AKI.
Association of urinary DKK3 with mortality
We explored the association of uDKK3/Cr with PICU mortality in Fig. 2 and Supplementary Table S5. Univariate analysis showed that the uDKK3/Cr was associated with mortality (OR = 4.290, 95% CI: 2.536–7.257, P < 0.001). In addition, the association remained significant after adjustment for BMI SDS, PRISM III score, AKI stage and sepsis (AOR = 2.213, 95% CI: 1.169–4.190, P = 0.015). The uDKK3/Cr was predictive of PICU mortality and displayed an AUC of 0.78 (P < 0.001), which was similar to the PRISM III score (AUC = 0.78, P < 0.001) in Fig. 3e. Adding the PRISM III score to the model that just included uDKK3/Cr was not superior to that of uDKK3/Cr alone for the prediction of mortality (▵AUC = 0.03, P = 0.484).
Moreover, the levels of uDKK3/Cr were divided into quartiles to further investigate the association of uDKK3/Cr with clinical outcomes. As presented in Fig. 4, the uDKK3/Cr yielded an unadjusted OR of AKI, severe AKI, sepsis, SA-AKI, and PICU mortality that was 5.804, 14.445, 7.698, 9.258, and 11.378 times as high in the highest quartile as in the reference, respectively.
The uDKK3/Cr (ng/mg) levels in quartiles 1, 2, 3, and 4 were <1.21, 1.21–2.62, 2.63–7.43, and >7.43, respectively. Quartile 1 was the reference group, except for SA-AKI, in which quartile 2 was used as a reference due to the absence of critically ill children with SA-AKI in quartile 1 group. AKI acute kidney injury, CI confidence interval, SA-AKI sepsis-associated AKI, uDKK3/Cr urinary dickkopf-3-to-creatinine ratio. *P < 0.05 vs. reference group, **P < 0.001 vs. reference group.
Discussion
To the best of our knowledge, this is the first study to explore the clinical utility of urinary DKK3 in an ICU cohort. Our data demonstrate that urinary DKK3 level upon admission to a general medical PICU was capable of early predicting AKI and PICU mortality and discriminating SA-AKI from other types of AKI.
DKK3 is a multifunctional protein that involves in cell differentiation, proliferation, apoptosis, and other cellular processes through the Wnt/β-catenin pathway, and implicates in the pathogenesis of a variety of diseases.29 DKK3 could activate or inhibit the Wnt/β-catenin pathway in vitro, depending on the cellular context.30,31 It has been found that activation of the Wnt/β-catenin pathway via inhibiting the expression of DKK3 suppresses apoptosis to ameliorate AKI in ischemia-reperfusion induced rat and cell AKI models.32 DKK3 is produced by renal tubular epithelial cells within the kidney, and it is secreted into the urine under acute or sustained tubular stress conditions.30 Although DKK3 is not expressed in the kidneys of healthy mice or detectable in the urine of healthy mice and healthy humans,30 a rise in the urinary DKK3 level has been discovered as a potential biomarker to monitor the progression of kidney diseases.14,33 In an observational cohort study conducted in an adult population, elevated urinary DKK3 concentrations before cardiac surgery were associated with an increased risk of AKI during hospitalization and decreased kidney function at discharge and long-term follow-up. To date, only a few studies have concentrated on the role of urinary DKK3 in AKI.14,34,35 These research works were specifically focused on AKI in adults within a specific clinical scenario, however, scarce research works have focused on the clinical value of urinary DKK3 in patients admitted to the ICU. To our knowledge, the cause and course of AKI can be highly variable in critically ill patients, and the immature kidney function of children differs from that of adults.19 Thus, this study was conducted in a heterogeneous PICU cohort to explore the clinical utility of urinary DKK3.
Our data showed that urinary DKK3 might be an early biomarker for AKI and severe AKI in a general PICU population. Many studies have been performed on the discovery of AKI biomarkers;11,36 however, currently used biomarkers only begin to increase after the occurrence of renal injury.14,37 Urinary DKK3 is considered to be different from these biomarkers, such as KIM-1 and NGAL, that allow an early detection instead of an early prediction before AKI.14 Schunk et al. suggested that preoperative urinary DKK3 has the ability to identify patients at high risk for postoperative AKI or persistent renal dysfunction.14 In this study, urine samples were collected in the first 24 h after PICU admission regardless of whether the diagnostic criteria for AKI had been met, and an increased level of urinary DKK3 is associated with a higher risk of AKI and severe AKI. Urinary DKK3 had an AUC of 0.70 for AKI and an AUC of 0.76 for severe AKI, indicating that urinary DKK3 is promising as an early discriminative biomarker for AKI and severe AKI in critically ill children, even those who have not met the criteria of AKI at the moment of sampling. However, the predictive ability of urinary DKK3 in the present study was a little lower than the postoperative result after cardiac surgery (AUC = 0.78).14 The difference in cohorts between these two studies could explain the disparity: one is an adult cohort undergoing cardiac surgery, while the other is a pediatric cohort admitted to PICU. Given the heterogeneity and dynamic nature of AKI, the discriminating ability of biomarkers is dependent strongly on the underlying conditions. In contrast, although some AKI events occurred several days after sample collection, which was likely to reduce the predictive accuracy of urinary DKK3 for AKI cases, urinary DKK3 had better discrimination in this study than the results reported previously in contrast-induced AKI in patients who received coronary angiography.34,35 The mild severity of AKI with none of the patients suffering from AKI stage 3 in the study by Seibert et al. (AUC = 0.61) might be a probable explanation for the discrepancy. In addition, all patients with AKI were complicated with hypertension before coronary angiography in this previous study,34 and some patients received preventive fluid admission before and after coronary angiography, which contributed to a transient increase in eGFR and then reduced the incidence of AKI. These influencing factors might result in a low discriminatory potential of urinary DKK3 in contrast-induced AKI.
Sepsis is the most common cause of AKI, concurrently, the incidence of SA-AKI among AKI cases in critically ill patients can be as high as approximately 50%.4,38 Critically ill patients with SA-AKI have a longer length of ICU stay and a higher ICU mortality rate versus AKI patients without sepsis.39,40 It is considered that DKK3 might be involved in the pathogenesis of sepsis through the Wnt/β-catenin pathway. Our study demonstrated that urinary DKK3 levels were associated with both sepsis and AKI, implying that the increases in urinary DKK3 levels due to sepsis and AKI are additive. Although the discriminative ability of urinary DKK3 for sepsis in our study was poor, urinary DKK3 had a robust relationship with SA-AKI and displayed an AUC of 0.80 for the discrimination, indicating that urinary DKK3 could be diagnostic of SA-AKI in critically ill children. Our data implied that DKK3 is secreted into urine by renal tubular epithelial cells after acute stress, which leads to higher levels of urinary DKK3 in critically ill children with AKI, including SA-AKI. Given the elevated DKK3 concentrations are attributable to sepsis and AKI, children with SA-AKI could have greater levels of urinary DKK3 than children with other types of AKI. Indeed, our finding further demonstrated that urinary DKK3 was capable of discriminating AKI cases in sepsis children and differentiating sepsis cases in AKI children, which emphasized an excellent ability for urinary DKK3 to discriminate SA-AKI from sepsis children without AKI or from other types of AKI. However, a larger-scale investigation is required.
It is of interest that urinary DKK3 was significantly associated with age, body weight, BMI, and BMI SDS in this study. DKK3 is expressed in the developing human kidney, suppressed in adult life, and re-expressed in pathological conditions, such as tubular stress.16,30,41 As kidney function is not mature in childhood, we speculate that the inverse association may reflect the process of kidney development. A previous study revealed a negative relationship between plasma DKK3 levels and BMI in a general population cohort.42 DKK3 seems to be expressed only in tubular epithelial cells within the kidney, but it is also expressed in other organs and tissues.43 In general, DKK3 is measurable in plasma but not in urine.30,43 It is still unclear whether urinary DKK3 is partially derived from filtered plasma DKK3 after glomerular injury. It is well known that AKI mainly manifests as tubular damage rather than glomerular damage. Although DKK3 has a predicted molecular weight of 38 kDa, its molecular weight could increase to 60–70 kDa through glycosylation.44 This may prevent plasma DKK3 from crossing the glomerular barrier easily. Hence, it is essential to conduct a future study to investigate the association between urinary DKK3 and plasma DKK3 levels in critically ill pediatric patients.
A positive correlation was found between urinary DKK3 levels and PRISM III score. The PRISM III score is a valid tool to evaluate the condition of critically ill children, and it has an excellent capacity to discriminate between survivors and non-survivors in the PICU cohort.20,45 Therefore, the correlation between urinary DKK3 and PRISM III score raised the question of whether urinary DKK3 is associated with adverse clinical outcomes in critically ill pediatric patients. In an observational study conducted on chronic obstructive pulmonary disease patients, urinary DKK3 was capable of identifying patients with worsening pulmonary function.46 However, rare studies have focused on the predictive value of urinary DKK3 on mortality in patients, especially in pediatric patients. We further defined the prognostic value of urinary DKK3 to confirm its prognostic significance. Our study demonstrated that urinary DKK3 is independently associated with PICU mortality, even after adjustment for BMI SDS, PRISM III score, AKI stage, and sepsis. Furthermore, the prognostic accuracy of DKK3 was similar to that of PRISM III score for PICU mortality, which indicated that urinary DKK3 may be utilized as an auxiliary tool to assess mortality risk for convenient use. The identification of risk factors for death in the early period of PICU stay might facilitate future intervention to prevent adverse outcomes.
The present study has some limitations. First, this study was a single-center study without a validation cohort, which may limit the generalizability of the results. Nevertheless, a prospective research design and a relatively large sample size could provide adequate power to evaluate the association between urinary DKK3 and clinical outcomes. Second, urine samples were only collected in the first 24 h after PICU admission instead of serial collections. The changes in urinary DKK3 values during the PICU stay were not assessed. In particular, it remains to be elucidated whether urinary DKK3 levels could reflect the clinical responses to treatment in children requiring intensive care. Third, as a common issue in the pediatric cohort, baseline sCr data were not available for most children in this study. We estimated the baseline sCr using the modified Schwartz estimating equation, which is a strategy that has been used in many pediatric AKI studies, to minimize errors as much as possible.25,26 Fourth, although sepsis is frequently complicated by AKI, we did not perform an etiological analysis for the remaining causes of AKI in this study. Whether urinary DKK3 plays an important role in diverse etiologies of AKI is still unclear. Further study is required to validate the effectiveness of urinary DKK3 in external cohorts and determine the clinical utility of urinary DKK3 in different causes of pediatric AKI.
In conclusion, urinary DKK3 was significantly associated with an increased risk for AKI, SA-AKI, and PICU mortality, even after adjustment for confounding factors. Urinary DKK3 upon admission to the PICU may be a potential biomarker for the early prediction of AKI, SA-AKI, and PICU mortality in critically ill children. Further studies are warranted to explore the effectiveness of urinary DKK3 in providing prognostic information in various pediatric cohorts.
Data availability
The data analyzed during the current study are available from the corresponding author on reasonable request.
References
James, M. T., Bhatt, M., Pannu, N. & Tonelli, M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 16, 193–205 (2020).
Kaddourah, A., Basu, R. K., Bagshaw, S. M., Goldstein, S. L. & Investigators, A. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625 (2018).
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ 364, k4891 (2019).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Kellum, J. A. et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am. J. Respir. Crit. Care Med. 193, 281–287 (2016).
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Ronco, C., Bellomo, R. & Kellum, J. Understanding renal functional reserve. Intensive Care Med. 43, 917–920 (2017).
Griffin, B. R., Gist, K. M. & Faubel, S. Current status of novel biomarkers for the diagnosis of acute kidney injury: a historical perspective. J. Intensive Care Med. 35, 415–424 (2020).
Pickkers, P. et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 47, 835–850 (2021).
Luft, F. C. Biomarkers and predicting acute kidney injury. Acta Physiol. (Oxf.) 231, e13479 (2021).
Albert, C. et al. Biomarker-guided risk assessment for acute kidney injury: time for clinical implementation? Ann. Lab. Med. 41, 1–15 (2021).
Li, Y. et al. DKK3 regulates cell proliferation, apoptosis and collagen synthesis in keloid fibroblasts via TGF-beta1/Smad signaling pathway. Biomed. Pharmacother. 91, 174–180 (2017).
Schunk, S. J. et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet 394, 488–496 (2019).
Vanmassenhove, J., Kielstein, J., Jörres, A. & Biesen, W. V. Management of patients at risk of acute kidney injury. Lancet 389, 2139–2151 (2017).
Schunk, S. J., Floege, J., Fliser, D. & Speer, T. WNT-beta-catenin signalling – a versatile player in kidney injury and repair. Nat. Rev. Nephrol. 17, 172–184 (2021).
Silva-Garcia, O., Valdez-Alarcon, J. J. & Baizabal-Aguirre, V. M. The Wnt/beta-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediators Inflamm. 2014, 310183 (2014).
Villar, J. et al. WNT/beta-catenin signaling is modulated by mechanical ventilation in an experimental model of acute lung injury. Intensive Care Med. 37, 1201–1209 (2011).
Rodieux, F., Wilbaux, M., van den Anker, J. N. & Pfister, M. Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin. Pharmacokinet. 54, 1183–1204 (2015).
Pollack, M. M., Patel, K. M. & Ruttimann, U. E. PRISM III: an updated Pediatric Risk of Mortality score. Crit. Care Med. 24, 743–752 (1996).
Li, H., Ji, C. Y., Zong, X. N. & Zhang, Y. Q. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi 47, 487–492 (2009).
Palevsky, P. M. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 61, 649–672 (2013).
Schwartz, G. J. et al. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 20, 629–637 (2009).
Targher, G. et al. Relationship between PNPLA3 rs738409 polymorphism and decreased kidney function in children with NAFLD. Hepatology 70, 142–153 (2019).
Myers, S. R. et al. Frequency and risk factors of acute kidney injury during diabetic ketoacidosis in children and association with neurocognitive outcomes. JAMA Netw. Open 3, e2025481 (2020).
Hursh, B. E., Ronsley, R., Islam, N., Mammen, C. & Panagiotopoulos, C. Acute kidney injury in children with type 1 diabetes hospitalized for diabetic ketoacidosis. JAMA Pediatr. 171, e170020 (2017).
Weiss, S. L. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 46, 10–67 (2020).
Matics, T. J. & Sanchez-Pinto, L. N. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 171, e172352 (2017).
Fang, X., Hu, J., Chen, Y., Shen, W. & Ke, B. Dickkopf-3: current knowledge in kidney diseases. Front Physiol. 11, 533344 (2020).
Federico, G. et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 1, e84916 (2016).
Nakamura, R. E. & Hackam, A. S. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 28, 232–242 (2010).
Zhu, X., Li, W. & Li, H. miR-214 ameliorates acute kidney injury via targeting DKK3 and activating of Wnt/β-catenin signaling pathway. Biol. Res. 51, 31 (2018).
Zewinger, S. et al. Dickkopf-3 (DKK3) in urine identifies patients with short-term risk of eGFR loss. J. Am. Soc. Nephrol. 29, 2722–2733 (2018).
Seibert, F. S. et al. Dickkopf-3 in the prediction of contrast media induced acute kidney injury. J. Nephrol. 34, 821–828 (2021).
Roscigno, G. et al. Urinary Dickkopf-3 and contrast-associated kidney damage. J. Am. Coll. Cardiol. 77, 2667–2676 (2021).
Wen, Y. & Parikh, C. R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab Sci. 58, 354–368 (2021).
Hayek, S. S. et al. Soluble urokinase receptor and acute kidney injury. N. Engl. J. Med. 382, 416–426 (2020).
Pettila, V. & Bellomo, R. Understanding acute kidney injury in sepsis. Intensive Care Med. 40, 1018–1020 (2014).
Shum, H. P., Kong, H. H., Chan, K. C., Yan, W. W. & Chan, T. M. Septic acute kidney injury in critically ill patients – a single-center study on its incidence, clinical characteristics, and outcome predictors. Ren. Fail 38, 706–716 (2016).
Bagshaw, S. M., George, C. & Bellomo, R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit. Care 12, R47 (2008).
Monaghan, A. P. et al. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech. Dev. 87, 45–56 (1999).
Piek, A. et al. The emerging plasma biomarker Dickkopf-3 (DKK3) and its association with renal and cardiovascular disease in the general population. Sci. Rep. 11, 8642 (2021).
Schunk, S. J., Speer, T., Petrakis, I. & Fliser, D. Dickkopf 3-a novel biomarker of the ‘kidney injury continuum’. Nephrol. Dial. Transplant. 36, 761–767 (2021).
Forsdahl, S., Kiselev, Y., Hogseth, R., Mjelle, J. E. & Mikkola, I. Pax6 regulates the expression of Dkk3 in murine and human cell lines, and altered responses to Wnt signaling are shown in FlpIn-3T3 cells stably expressing either the Pax6 or the Pax6(5a) isoform. PLoS One 9, e102559 (2014).
Zhang, L. et al. Performance of PRISM III, PELOD-2, and P-MODS scores in two pediatric intensive care units in China. Front Pediatr. 9, 626165 (2021).
Schunk, S. J. et al. Measurement of urinary Dickkopf-3 uncovered silent progressive kidney injury in patients with chronic obstructive pulmonary disease. Kidney Int. 100, 1081–1091 (2021).
Funding
This work was supported by grants from the National Natural Science Foundation of China (81971432), Jiangsu Province Science and Technology Support Program (BE2020660), and Key Talent of Women’s and Children’s Health of Jiangsu Province (FRC201738). The funders had no role in study design, data collection, preparation of the manuscript, and decision to publish.
Author information
Authors and Affiliations
Contributions
J.H. performed the data analysis and drafted the manuscript. Y.Z. performed the experiments. H.H. and Y.K. participated in the data analysis and interpretation. J.C. and Z.B. participated in collecting the data and samples. X.L. coordinated and supervised data collection and carried out the initial interpretation of data. Y.L. had primary responsibility for study design, performing the experiments, data analysis, interpretation of data, and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Children’s Hospital of Soochow University and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from each participating individual’s guardian.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, J., Zhou, Y., Huang, H. et al. Prediction of urinary dickkopf-3 for AKI, sepsis-associated AKI, and PICU mortality in children. Pediatr Res 93, 1651–1658 (2023). https://doi.org/10.1038/s41390-022-02269-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02269-4
This article is cited by
-
The role of urinary Dickkopf-3 in the prediction of acute kidney injury: a systematic review meta-analysis
International Urology and Nephrology (2023)