Abstract
Background
Studies have suggested a link between prenatal maternal acetaminophen use and adverse developmental outcomes in children. However, there exists a knowledge gap regarding overall cognitive development and use of acetaminophen, especially concerning the timing of use in pregnancy. This study aimed to characterize the relationship between maternal acetaminophen use and cognitive development at 4 years.
Methods
This analysis included data collected throughout pregnancy and delivery from women in the Ontario Birth Study prospective cohort from 2013 to 2019 and from the NIH Toolbox Early Childhood Cognition battery administered to 4-year-old children between 2018 and 2021 (n = 436). The exposure was maternal acetaminophen use and the primary outcome was a cognition composite score. The relationship between exposure and outcome was determined using Poisson regression with a robust error variance.
Results
We did not observe any association between maternal acetaminophen intake any time before or during pregnancy and low cognition composite score of offspring. The IRR of suboptimal overall cognition was 1.38 (0.78–2.45), 1.22 (0.67–2.22), 0.80 (0.44–1.47), and 1.56 (0.74–3.29) for maternal use of acetaminophen before, in early, late, or overall pregnancy, respectively.
Conclusion
Current data do not provide evidence to support a relationship of maternal acetaminophen use during pregnancy with adverse cognitive effects at 4 years.
Impact
-
Acetaminophen use during pregnancy may influence the risk of child neurocognitive disorders, but there is conflicting evidence of its relationship to sub-clinical measures of cognitive development such as executive function.
-
The study design allowed us to examine the role of timing of acetaminophen use in its relationship with cognitive development, based on a validated and standardized tablet-administered instrument for children, instead of a teacher or parent report.
-
We did not observe a clear relationship between maternal acetaminophen use at different timepoints during pregnancy and child cognitive development.
Similar content being viewed by others
Introduction
Acetaminophen is the only analgesic recommended by doctors for pregnant women, and 56–70% of mothers report use during pregnancy.1,2 Several studies have examined the relationship of maternal acetaminophen use during pregnancy with child neurodevelopment and cognitive outcomes, including ADHD and cerebral palsy.2,3,4,5,6,7 These include studies from the 2004 and 2015 Pelotas Birth cohorts, the Avon Longitudinal Study of Parents and Children, and the Norwegian Mother and Child cohort, among others.3,4,5,6,7 The results from these studies have varied, as some identified increased risk of hyper-kinetic disorders or cerebral palsy2,8,9, and others found no associations.10 Recent studies have also suggested that prenatal exposure to acetaminophen could have an adverse impact on child executive function: one study from the American Project Viva cohort utilized the Behavior Rating Inventory of Executive Function and the Strengths and Difficulties Questionnaire and reported that children (median 8 years old) who were exposed to acetaminophen prenatally performed worse than children who were not exposed.5 In contrast, another study found no association between prenatal acetaminophen use and child neurodevelopmental measures at 24 months.6 Despite the wealth of new research in this area, the implications of acetaminophen use on child cognition are largely inconclusive. In addition, studies have often assessed child cognition using parent and teacher-reported measures.11 Our study aims to provide further insight into this potential relationship using a standardized instrument completed by the child at 4 years of age.
Methods
Study design
The Ontario Birth Study (OBS) was initiated in 2013 as a prospective pregnancy and birth cohort. Women who seek prenatal care at Mount Sinai Hospital in Toronto are recruited between 11 and 14 weeks of gestation. Participants are asked to complete questionnaires at 12–16 and 28–32 weeks gestational age, as well as at 6–10 weeks postpartum. Detailed information regarding study procedures was reported previously.12 Recruitment is currently ongoing, and this paper reports on clinical and lifestyle data that were collected from 1955 pregnancies from 2013 to 2019.
In February 2018, the Ontario Birth Study-Kids (OBS-Kids) began recruiting OBS participants who consented to future contact and who had a surviving OBS child. Participants are asked to complete questionnaires when the child is 8, 24, and 36 months old, as well as at 4 years of age when children are asked to complete the NIH Toolbox® for Assessment of Neurological and Behavioural Function (NIH Toolbox) Early Childhood Cognition Battery.13
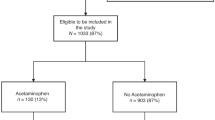
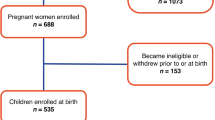
OBS mothers are eligible to participate in the OBS-Kids follow-up with consent to future contact, completion of one or both prenatal questionnaires, and live birth. Figure 1 shows the inclusions and exclusions of the study participants from the OBS cohort to the 4-year follow-up assessment available for this analysis. Of the 1955 OBS mother–child dyads that were eligible for recruitment to the OBS-Kids study, 1312 had children reaching 4 years old and 1045 were sent invitations by March 2021. A total of 616 of these mother–child dyads completed the 4-year follow-up by this date: 180 dyads were excluded due to missing cognitive data or missing acetaminophen data, and consequently, 436 dyads were included in this analysis. Reasons for non-completion of the home visit include family relocation or preference for a phone interview over a home visit. In March 2020, the COVID-19 pandemic resulted in widespread lockdown and restrictions on in-person research activities, and this led to approximately 20% of otherwise eligible children at 4-year follow-up not being able to complete the cognition portion of the NIH Toolbox before the restrictions were lifted.
This study was approved by the Research Ethics Board at the Mount Sinai Hospital.
Exposure
Information on prenatal acetaminophen exposure was collected from participants using the prenatal questionnaires, as illustrated in Supplementary Fig. 1. Participants were asked about the frequency of acetaminophen use within the 3 months prior to pregnancy, early pregnancy (first 12–16 weeks), and later pregnancy (3 months prior to the second questionnaire administered at 28–32 weeks). Response options for each of these three timepoints were never, less than 1 per month, 1–3 days per month, 1–3 days per week, 4–6 days per week, or every day. Overall use, which was defined as use at any time before or during pregnancy, was derived from these three items. Participants were defined as having used acetaminophen at a time point if they reported use for at least one day per month during that period.
Outcome
The NIH Toolbox Early Childhood Cognition battery was administered using a touch-screen tablet to children from index pregnancies aged 4 years between February 2018 and March 2021. The battery consists of the Picture Vocabulary Test, the Flanker Inhibitory Control Test, the Dimensional Change Card Sort Test, and the Picture Sequence Memory Test. These tests assess language development, inhibitory control and attention, cognitive flexibility, and episodic memory, respectively.13 The NIH Toolbox was validated for children in an American study with commonly used neuropsychological tests selected for validation based on each construct for the 3–6-year-old cohort. Correlations between NIH Toolbox measures and corresponding selected validation measures were calculated to assess convergent validity.13 The NIH Toolbox has also been validated in specific pediatric populations.14
Children remain unassisted during the test but are supervised by trained study personnel and usually accompanied by a parent. Scores for each individual test, as well as a composite score representing overall cognition, are calculated automatically based on methods previously reported.15 In some cases when only one test was missing, a composite score was derived based on the mean of the scores from the three remaining tests (n = 22). In the event that a child completed no more than two of the four tests, composite scores were not calculated (n = 4). For the four individual tests and the composite cognition score, standardized age-corrected scores with a mean of 100 and a standard deviation of 15 were used in the analysis.15 Higher scores represent better performance on the tests.
Statistical analysis
Cognition score categories were defined using a threshold at the 10th percentile of the sample population, resulting in a binary variable where children who scored below the threshold were considered suboptimal. These categories were regressed on acetaminophen intake using Poisson regression with a robust error variance to estimate incidence rate ratios (IRR) and 95% confidence intervals (CI). Models were adjusted for a priori selected potential confounders based on previously reported factors associated with child cognition scores, including maternal characteristics such as age, ethnicity, education, prenatal alcohol use, and child’s sex.16 Maternal smoking during pregnancy was not included in the regression model because only two mothers reported smoking at least one cigarette during pregnancy and none reported daily use. Preterm birth was not included in the model due to its low prevalence in the study population (6%).
Missing data
Missing values of continuous variables were imputed based on the median of the cohort, and missing values of binary variables were assigned with 50% probability of belonging to either of the dichotomous categories for alcohol consumption during pregnancy (n = 57), education (n = 15), and ethnicity (n = 10). Effectively, this is uninformative imputation that does not affect the estimates of the main variable of interest, but retains the sample size and statistical stability. Excluding these observations did not meaningfully alter the results.
Sensitivity analyses
To assess whether other prenatal and child-related factors could potentially be confounders, we conducted a secondary analysis of acetaminophen use with a model containing the following variables as covariates: maternal BMI, maternal fever during pregnancy, maternal smoking during pregnancy, antidepressant use in mid-pregnancy, depression and anxiety symptoms in mid-pregnancy (EPDS and PHQ-4), antibiotic use in mid-pregnancy, child’s gestational age at birth, child’s ethnicity, and household income. The results of this secondary analysis are illustrated in Supplementary Table 2. To identify any association with higher frequency use of acetaminophen, a secondary analysis was conducted where mothers were considered as exposed if they reported using acetaminophen at least one day per week during at least one of the defined timepoints. The results of this analysis are presented in Supplementary Table 3. To determine whether outcome definition could have affected the results, a secondary analysis was performed using a threshold at one standard deviation below the mean of cognition scores.
Results
Characteristics of the study sample are presented in Table 1. Mothers were predominately of European descent (71%) and had a bachelor’s degree or higher (85%). Alcohol consumption, defined as more than one drink per week, was common in the 3 months before pregnancy (68%), but less so during pregnancy (45%). It should be noted that consumption during pregnancy included mothers who drank before pregnancy and reported stopping less than two weeks before pregnancy was discovered, as this is likely to include the first few weeks of pregnancy.
Overall acetaminophen use was common (69%), whereas 38%, 36%, and 43% of mothers reported use before pregnancy, in early pregnancy, and in late pregnancy, respectively. High-frequency acetaminophen use was less common (36%), and fewer mothers reported use before pregnancy (8%), in early pregnancy (16%), and in late pregnancy (18%). Children born to mothers who reported acetaminophen use did not perform significantly differently on any cognitive test except for the picture vocabulary test, where they performed slightly better than those who were not exposed at the late pregnancy questionnaire (covering 3 months prior to 28–32 weeks) (IRR: 0.65, 95% CI 0.45, 0.94, p = 0.02; Table 2). There was no meaningful difference in results based on the secondary analyses, including per standard deviation, additional potential confounders (Supplementary Table 2), or high-frequency use (Supplementary Table 3).
To assess whether there is any potential bias from loss to follow-up, we compared key demographic and risk factors between those without versus with cognitive data among those who are eligible for OBS 4-year follow-up components. The two groups were mostly comparable, although acetaminophen use was slightly higher among those with cognitive data (Supplementary Table 1).
Discussion
In this analysis, we did not observe that maternal acetaminophen use is related to child cognition. This finding is in contrast to a previous study that suggested that prenatal acetaminophen use is associated with lower cognitive scores in a cohort of children with a median age of 8 years.5 Several other studies have provided evidence for a relationship between acetaminophen use and poorer neurodevelopmental outcomes in other pediatric age groups,2,4,8 though these outcomes have generally been behavioral in nature, rather than cognitive. Relationships observed in a number of studies are also greatly attenuated by accounting for confounders.7,11,17 It should be noted, however, that many of the studies investigating this relationship to date have not been conducted in the same age group as the current study; thus, direct comparison to the findings of other studies is not possible. Our findings are supported by studies that have not identified any relationship between acetaminophen use and child cognition.3,6
The main limitation of this study is the limited statistical power due to the low prevalence of underperforming children in the study sample. The positive association with picture vocabulary where CIs excluded one may have occurred by chance given the low sample size. High-frequency acetaminophen use was not common among the mothers in our cohort; thus, we were unable to assess heavier use. In addition, our study did not collect indication for acetaminophen use; thus, we were unable to include it in our models. Furthermore, acetaminophen use after 32 weeks was not assessed by our study; thus, the prevalence of overall and later pregnancy acetaminophen use may be underestimated. It is important to note that this population has a high proportion of university-educated white women, thus limiting generalizability to populations with similar demographics. Finally, the considerable reduction in numbers from eligible mother–child dyads to those who completed the cognitive assessment may introduce selection bias. However, the comparison between those with versus without cognitive data did not indicate meaningful differences related to key factors, and to some extent, the non-completion was due to overall COVID restriction, instead of individual selection.
Strengths of this study include its prospective design, wherein acetaminophen use was assessed prior to the outcome at two different timepoints throughout pregnancy and closer to the time of use than in studies that assessed prenatal use after birth.6,7,11 Despite this, it is important to note that the misclassification of exposure due to recall error is possible. In addition, we assessed child cognition using the validated NIH Toolbox,13 which allows for direct assessment of neurodevelopment. It is performed by the child, unlike parent- or teacher-reported tools, minimizing the probability of misclassification from surrogate respondents. Our study also accounts for multiple confounders, a common threat to validity in recent studies.17
Conclusions
The potential effect of maternal acetaminophen use on child health and development remains inconclusive. Further research should seek to validate and expand recent findings to understand the impact of indication of acetaminophen use on the relationship between acetaminophen and child cognition.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Werler, M. M., Mitchell, A. A., Hernandez-Diaz, S. & Honein, M. A. Use of over-the-counter medications during pregnancy. Am. J. Obstet. Gynecol. 193, 771–777 (2005).
Liew, Z., Ritz, B., Rebordosa, C., Lee, P. C. & Olsen, J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 168, 313–320 (2014).
Bertoldi, A. D. et al. Associations of acetaminophen use during pregnancy and the first year of life with neurodevelopment in early childhood. Paediatr. Perinat. Epidemiol. 34, 267–277 (2020).
Golding, J. et al. Associations between paracetamol (acetaminophen) intake between 18 and 32 weeks gestation and neurocognitive outcomes in the child: a longitudinal cohort study. Paediatr. Perinat. Epidemiol. 34, 257–266 (2020).
Rifas-Shiman, S. L. et al. Associations of prenatal or infant exposure to acetaminophen or ibuprofen with mid-childhood executive function and behaviour. Paediatr. Perinat. Epidemiol. 34, 287–298 (2020).
Tovo-Rodrigues, L. et al. Low neurodevelopmental performance and behavioural/emotional problems at 24 and 48 months in Brazilian children exposed to acetaminophen during foetal development. Paediatr. Perinat. Epidemiol. 34, 278–286 (2020).
Trønnes, J. N., Wood, M., Lupattelli, A., Ystrom, E. & Nordeng, H. Prenatal paracetamol exposure and neurodevelopmental outcomes in preschool-aged children. Paediatr. Perinat. Epidemiol. 34, 247–256 (2020).
Thompson, J. M. D., Waldie, K. E., Wall, C. R., Murphy, R. & Mitchell, E. A. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS ONE 9, e108210 (2014).
Petersen, T. G. et al. Use of paracetamol, ibuprofen or aspirin in pregnancy and risk of cerebral palsy in the child. Int. J. Epidemiol. 47, 121–130 (2018).
Hjorth, S. et al. Prenatal exposure to non-steroidal anti-inflammatory drugs and risk of attention-deficit/hyperactivity disorder: a follow-up study in the Norwegian mother, father and child cohort. Pharmacoepidemiol. Drug Saf. 30, 1380–1390 (2021).
Parker, S. E., Collett, B. R. & Werler, M. M. Maternal acetaminophen use during pregnancy and childhood behavioural problems: discrepancies between mother- and teacher-reported outcomes. Paediatr. Perinat. Epidemiol. 34, 299–308 (2020).
Anderson, L. N. et al. The Ontario Birth Study: a prospective pregnancy cohort study integrating perinatal research into clinical care. Paediatr. Perinat. Epidemiol. 32, 290–301 (2018).
Weintraub, S. et al. I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr. Soc. Res Child Dev. 78, 1–15, https://pubmed.ncbi.nlm.nih.gov/23952199 (2013).
Downes, M. et al. Executive function in children with sickle cell anemia on transfusion: NIH toolbox utility in the clinical context. Clin. Neuropsychol. 1–16 (2020).
National Institutes of Health. NIH Toolbox® Scoring and Interpretation Guide. 17–19 (2016).
Meadows, S. Chapter 5: causes of change and variation in neurodevelopment. In: The Child as a Thinker 313–408 (New York, Routledge, 2006).
Masarwa, R., Platt, R.W. & Filion, K. B. Acetaminophen use during pregnancy and the risk of attention deficit hyperactivity disorder: a causal association or bias? Paediatr. Perinat. Epidemiol. 34, 309–317 (2020).
Acknowledgements
We thank the participants and staff of the Ontario Birth Study and the Ontario Birth Study-Kids.
Funding
This work was supported by Canadian Institutes of Health Research [CU3 160313] (to J.A.K.), Canadian Institutes of Health Research (CIHR) [FDN 143262] (to S.J.L.), Cameron Holcombe Wilson Research Chair (to R.D.L.), Lunenfeld-Tanenbaum Research Institute and the Department of Obstetrics and Gynecology, Sinai Health System, Mount Sinai Hospital Foundation, and CIHR Canada Research Chair (to R.J.H.).
Author information
Authors and Affiliations
Contributions
J.M.L., R.J.H., and J.A.K. conceived and designed the study, acquired and analyzed the data, and drafted and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
All mothers signed a written, informed consent for themselves and their child’s participation in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lye, J.M., Knight, J.A., Arneja, J. et al. Maternal acetaminophen use and cognitive development at 4 years: the Ontario Birth Study. Pediatr Res 93, 959–963 (2023). https://doi.org/10.1038/s41390-022-02182-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02182-w