Abstract
Background
Brain injury and neurodevelopmental impairment remain a concern in children with complex congenital heart disease (CHD). A practice guideline on neuromonitoring, neuroimaging, and neurodevelopmental follow-up in CHD patients undergoing cardiopulmonary bypass surgery is lacking. The aim of this survey was to systematically evaluate the current practice in centers across Europe.
Methods
An online-based structured survey was sent to pediatric cardiac surgical centers across Europe between April 2019 and June 2020. Results were summarized by descriptive statistics.
Results
Valid responses were received by 25 European centers, of which 23 completed the questionnaire to the last page. Near-infrared spectroscopy was the most commonly used neuromonitoring modality used in 64, 80, and 72% preoperatively, intraoperatively, and postoperatively, respectively. Neuroimaging was most commonly performed by means of cranial ultrasound in 96 and 84% preoperatively and postoperatively, respectively. Magnetic resonance imaging was obtained in 72 and 44% preoperatively and postoperatively, respectively, but was predominantly reserved for clinically symptomatic patients (preoperatively 67%, postoperatively 64%). Neurodevelopmental follow-up was implemented in 40% of centers and planned in 24%.
Conclusions
Heterogeneity in perioperative neuromonitoring and neuroimaging practice in CHD in centers across Europe is large. The need for neurodevelopmental follow-up has been recognized. A clear practice guideline is urgently needed.
Impact
-
There is large heterogeneity in neuromonitoring, neuroimaging, and neurodevelopmental follow-up practices among European centers caring for neonates with complex congenital heart disease.
-
This study provides a systematic evaluation of the current neuromonitoring, neuroimaging, and neurodevelopmental follow-up practice in Europe.
-
The results of this survey may serve as the basis for developing a clear practice guideline that could help to early detect and prevent neurological and neurodevelopmental sequelae in neonates with complex congenital heart disease.
Similar content being viewed by others
Introduction
In Europe, the prevalence of congenital heart disease (CHD) is 8 per 1000 live births.1 Over the past decades, survival rates of children with complex CHD have significantly improved due to advances in neonatal and intensive care medicine and surgical techniques.2 However, this patient population continues to be at significant risk for neurodevelopmental sequelae throughout their life course3,4 This may exert long-lasting adverse effects on the individuals, their families, and society.5
As potential underlying mechanisms, the risk for abnormal intrauterine brain development and neonatal brain injury has long been recognized in this population, with small focal strokes and punctate white matter lesions being the most frequently observed types of brain lesions.6,7,8,9,10,11,12,13,14 Numerous studies have shown that neuroimaging and neuromonitoring techniques can be valuable tools in the early detection of neonatal brain injury, identification of risk for later neurodevelopmental sequelae, and help optimize perioperative care to attenuate and modify risk factors.11,15,16,17,18 Despite this evidence, there is no clear practice guideline or standardized protocol on how to monitor neonates with complex CHD in the vulnerable perioperative period.
In contrast, there is consensus on the need for neurodevelopmental follow-up for children with complex CHD and recently recommendations for comprehensive neurodevelopmental follow-up programs for preschool19 and school-age children20 have been issued. However, little is known about the implementation of these guidelines in neonatal cardiac surgery centers across Europe.
The European Association Brain and Congenital Heart Disease (ABC) Consortium is a multicenter, multidisciplinary group, financially supported by the European Society for Paediatric Research, that aims to promote research in the field of brain development in CHD children and, thereby, improve the neurodevelopmental outcome of infants with severe CHD.21 With this survey, we aimed to obtain detailed information on neuromonitoring practices in European centers caring for neonates with CHD. We designed and distributed an online survey inquiring about the use and timing of neuromonitoring and neuroimaging tools in pediatric cardiac surgical centers. Furthermore, we obtained information on the implementation of neurodevelopmental follow-up programs and the general interest in a European neurodevelopmental outcome registry. The results of this survey may serve as the basis for the development of an expert panel recommendation on neuromonitoring, neuroimaging, and neurodevelopmental follow-up in CHD patients undergoing neonatal cardiac surgery.
Methods
We conducted a structured online survey on the current implementation of neuromonitoring, neuroimaging, and neurodevelopmental follow-up in infants with congenital heart disease who require cardiopulmonary bypass surgery. The questionnaire included details on preoperative, intraoperative, and postoperative neuromonitoring modalities, preoperative and postoperative neuroimaging, measurement of postoperative biochemical markers, and the implementation of neurodevelopmental follow-up and data entry into a register. Furthermore, we investigated the interest in participating in a European neurodevelopmental outcome registry.
The survey was constructed and designed with the professional and freely accessible online survey tool available on https://www.soscisurvey.de/ (last accessed March 2021). As there was no standardized questionnaire available on perioperative neuromonitoring and neuroimaging, the content and design of the questionnaire were jointly agreed upon by consensus from the European ABC Consortium. Furthermore, we piloted and modified the questionnaire within our study group. The questionnaire was directly distributed to pediatric cardiac surgical centers, the European Brain Club, the Association for European Pediatric and Congenital Cardiology (AEPC), and personal contacts by members of the consortium. Furthermore, the questionnaire was sent to all members of the European Society for Paediatric Research via the monthly newsletter. The survey can be found as Supplementary Fig. 1. Respondents were asked to voluntarily identify their institution and provide contact information for potential follow-up questions. If multiple reports of the same institution were identifiable, centers were contacted to reach a consensus on their entries, or the most complete entry was kept. Data were collected between April 2019 and June 2020. Only valid questionnaire responses were considered for further analysis. Valid entries were defined as respondents reaching at least page 6 of 19, i.e., at least reaching questions on preoperative neuromonitoring. Furthermore, we defined a rate of <60% missing entries as a valid response cut-off to exclude cases in which respondents just viewed the questionnaire without filling it in. This research project was deemed exempt from the requirement for approval by the ethics committee.
Statistical analysis
The evaluation of the survey results was of descriptive nature. Categorical variables were reported as frequencies and percentages. Free text comments were qualitatively summarized.
Results
General characteristics of the responding centers
During the survey period, 25 European centers provided a valid response to the questionnaire (n = 22 excluded for aborting the questionnaire before page 6; n = 3 excluded for >60% missing entries). Among these, 23/25 centers completed the questionnaire to the last page. The completion rate, i.e., the number of complete questionnaires divided by started questionnaires measured by clicks on the link was 46% (23/50). For the geographical distribution of the 25 centers across Europe who provided a valid entry, see Fig. 1. At the centers, the questionnaire was filled in by pediatric cardiologists in 40% (10/25), neonatologists in 20% (5/25), others (e.g., pediatric neurologists, pediatric radiologists, developmental pediatricians, pediatric and congenital cardiac surgeons) in 12% (3/25), and pediatric intensivists in 8% (2/25) of cases. Multiple subspecialties were indicated by 20% (5/25).
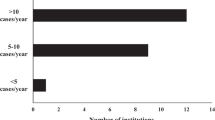
The majority of centers (56% (14/25)) indicated that preoperative care in infants with CHD was carried out in different units depending on the age and clinical status of the patients. In 20% (5/25), treatment was carried out in a neonatology unit, in 16% (4/25) in a cardiology unit, and in 8% (2/25) in a pediatric intensive care unit. Regardless of the type of unit, 88% (22/25) of centers identified their unit as an intermediate or intensive care ward. In 32% (8/25) of responding centers >250 pediatric cardiac surgeries (age 0–16 years) were performed each year (Fig. 2a). In contrast, only 16% (4/25) of centers performed >100 neonatal cardiac surgeries with cardiopulmonary bypass surgery (<28 days of life) each year, whereas 24% (6/25) of centers performed <25 neonatal surgeries each year (Fig. 2b). The majority of centers (72% (18/25)) indicated performing the hybrid procedure (i.e., stenting of the arterial duct and banding of the pulmonary arteries22) in neonates with hypoplastic left heart syndrome in <25 neonates per year. In 16% (4/25), >25 hybrid procedures per year were performed, whereas 12% (3/25) did not know or answer the question.
Preoperative neuromonitoring practice
Regarding their preoperative neuromonitoring practice for neonates prior to cardiopulmonary bypass surgery, the majority used near-infrared spectroscopy (NIRS) 64% (16/25), while only 32% (8/25) used amplitude-integrated electroencephalography (aEEG) and 12% (3/25) used continuous video electroencephalography (cEEG) (Fig. 3a). Furthermore, 28% (7/25) of the responding centers indicated that no neuromonitoring tool was used prior to neonatal cardiac surgery. When using NIRS, a neonatal sensor as opposed to a pediatric/adult sensor was most frequently used (93.8% (15/16)). Among centers using aEEG, 2-channel aEEG as opposed to 1-channel aEEG was used in 75% (6/8). In those centers which indicated that neonatal preoperative neuromonitoring was performed, it was most often performed on a routine clinical basis (72.2% (13/18)), or on clinical indication (22.2% (4/18)), and only in 5.6% (1/18) merely for research purposes. Most centers performed a preoperative neurological examination only in cases of clinical concern 56% (14/25). Only a few centers performed the examination on a clinical routine basis (8% (2/25)) or as part of a research protocol (16% (4/25)). The examination was most often performed by a child neurologist or neonatologist (36% (9/25) and 40% (10/25)).
Bar plot showing the percentages of preoperatively and postoperatively used neuromonitoring and neuroimaging tools. aEEG amplitude-integrated electroencephalography, NIRS near-infrared spectroscopy, EEG electroencephalography, CT computer tomography, CUS cranial ultrasound, MRI magnetic resonance imaging.
Preoperative neuroimaging practice
Preoperative neuroimaging was used in almost all centers (96% (24/25)). Cranial ultrasound was used by 96% (24/25), magnetic resonance imaging (MRI) by 72% (18/25), and computer tomography (CT) by 32% (8/25) of responding centers (Fig. 3b). In the 18 centers performing MRI, it was mostly done for clinically symptomatic patients (67% (12/18)). In 33% (6/18) of centers, preoperative MRI was performed routinely either as a clinical routine (11% (2/18)) or for research purposes (22% (4/18)). One of the centers performing clinical routine MRI indicated that this was done in all patients undergoing cardiopulmonary bypass surgery, whereas the other center specifically targeted patients with transposition of the great arteries, aortic arch interruption, or hypoplastic left heart syndrome. Only 17% (3/18) of centers reported that routine clinical or research MRI was also performed in the intensive care unit. In free-text comments, respondents specified that MRI in intensive care unit patients was reserved only for symptomatic patients due to limited resources. MRI was either performed using sedation (22% (4/18)), in natural sleep with a feed and wrap technique (11% (2/18)), using general anesthesia (17% (3/18)) or a combination of modalities depending on the clinical situation (50% (9/18)) (Fig. 4a). We did not further inquire about the circumstances under which cranial ultrasound or CT was performed.
Intraoperative neuromonitoring practice
Intraoperative neuromonitoring was performed using NIRS in 80% (20/25), aEEG in 16% (4/25), bispectral index in 8% (2/25), and cEEG in 4% (1/25). No intraoperative neuromonitoring was performed in 8% (2/25), and no response was given by 12% (3/25) of questionnaire participants. The majority of centers did not administer neuroprotective agents (e.g., steroids, allopurinol, nitric oxide, mannitol, corticosteroids) (64% (16/25)) intraoperatively, 8% (2/25) did not know and 8% (2/25) did not answer the question.
Postoperative measurement of biochemical markers of neuronal injury
Postoperatively, most centers did not measure biomarkers of neuronal injury (64% (16/25)) or did not know (8% (2/25)) or answer the question (8% (2/25)). Only a few centers indicated measuring parameters such as neuron-specific enolase, protein S100, or glial fibrillary acidic protein (20% (5/25)) to assess neuronal injury post-surgery.
Postoperative neuromonitoring practice
At least one method of postoperative neuromonitoring was utilized in 72% (18/25) of centers, most commonly NIRS (72% (18/25)) and aEEG (20% (5/25) (Fig. 3c). Both tools in combination were used in 20% (5/25) of centers. A proportion of 20% (5/25) said they did not perform postoperative neuromonitoring or did not provide a response (8% (2/25)). Half of the respondents (48% (12/25)) indicated that a postoperative neurological examination was only performed in patients with clinical concerns. Furthermore, 24% (6/25) of centers indicated that no neurological examination was being routinely performed. A proportion of 20% (5/25) of responding centers indicated that they did perform a neurological examination, which was done in 80% (4/5) as part of a research protocol and only in one center (20% (1/5)) as part of clinical routine. 8% (2/25) did not respond to the question.
Postoperative neuroimaging practice
Postoperatively, the majority of responding centers (88% (22/25)) used at least one neuroimaging modality, only 4% (1/25) did not use any neuroimaging or did not respond to the question (8% (2/25)). Cranial ultrasound was used by 84% (21/25), MRI by 44% (11/25), and CT by 20% (5/25) of centers (Fig. 3c). Of those centers performing MRI postoperatively, it was most commonly acquired in symptomatic patients only (64% (7/11)), followed by a combination of symptomatic patients and based on a research protocol (18% (2/11)). Only one center performed MRI only for research purposes or as part of clinical routine respectively (9% (1/11)). Specifically, susceptibility weighted images to detect potential hemorrhagic lesions were acquired in 27% (3/11) of the centers, whereas 18% (2/11) additionally acquired venograms to detect sinovenous thromboses. A proportion of 27% (3/11) of centers performed postoperative MRI using general anesthesia, 18% (2/11) used sedation or a combination of natural sleep, sedation, and general anesthesia in 55% (6/11) (Fig. 4b). We did not further inquire about the circumstances under which cranial ultrasound or CT was performed.
Neurodevelopmental follow-up
Almost half of the responding centers (40% (10/25)) indicated having a follow-up program in place for children with CHD after cardiopulmonary bypass surgery, and 24% (6/25) were planning to implement one. In contrast, 28% (7/25) of centers did not have a follow-up program and 8% (2/25) did not respond to the question. Follow-up data were collected in a register in 32% (8/25); 32% (8/25) did not collect follow-up information and the remaining centers (36% (9/25)) did not respond to the question. The majority of centers (88% (22/25)) said they were interested in joining a European neurodevelopmental outcome register for neonates with CHD.
Discussion
In this European survey on perioperative neuromonitoring, neuroimaging, and neurodevelopmental follow-up in neonates undergoing cardiopulmonary bypass surgery for complex CHD, we found evidence for very heterogeneous practices in cardiac surgical centers across Europe.
Preoperatively, intraoperatively, and postoperatively NIRS was implemented in 2/3 of responding centers and was thus the most commonly used tool for neuromonitoring at all three inquired time points. Cardiac surgery is known to be associated intraoperatively and postoperatively with altered cerebral hemodynamic evident on NIRS monitoring.17 While NIRS is a valuable tool to monitor these perioperative changes and guide perioperative and intraoperative intensive care management, there are conflicting results on the predictive values of those parameters for brain injury and later neurodevelopment as reviewed by Zaleski and colleagues17 and reported in recent publications.23
In contrast, aEEG was only used in 20–32% of centers in the perioperative period. However, cerebral function monitoring by means of aEEG is recommended in neonates undergoing early surgery for CHD for surveillance and treatment of seizures.24 Furthermore, studies have shown that beyond seizure detection aEEG can inform the intensive care team about the risk for neonatal brain injury and long-term outcome prognosis. Preoperatively, an abnormal background pattern on aEEG was associated with neonatal brain injury,25 whereas in another study postoperative abnormal brain activity on aEEG (i.e., abnormal background pattern or ictal discharges) was associated with a fourfold increase in the risk for postoperative brain injury.15 While the predictive value of aEEG for the neurodevelopmental outcome has been well established in the population of infants with hypoxic–ischemic encephalopathy, first evidence showed a promising predictive value also in the postoperative cardiac population. Postoperatively, delayed recovery of background pattern on aEEG and lack of return to normal sleep–wake cycling has been shown to be associated with poor neurodevelopmental and cognitive outcomes at 2 and 4 years of age.16,26 A more systematic use and widespread implementation of this technique in perioperative infants with CHD could help to further improve the diagnostic and prognostic leverage of this tool.
Almost all centers used preoperative neuroimaging, with cranial ultrasound and MRI as the most commonly used modalities. However, MRI was predominantly only used in clinically symptomatic patients and rarely in patients cared for in the intensive care unit. The same was found for postoperative neuroimaging practices. Nevertheless, there is a large body of evidence demonstrating that perioperative brain injury is common in CHD21 and is clinically silent in the majority of cases.14 Additionally, studies have linked MRI-detected perioperative brain lesions with later adverse neurodevelopmental outcome18,27 underlining the potential predictive value of the tool. Furthermore, patients that require prolonged preoperative or postoperative care in an intensive care unit might be at the highest risk for neonatal brain injury, which can be missed, if a timely MRI is not available to these patients.21,28 Thus, the routine implementation of MRI-based neuroimaging preoperatively or postoperatively seems of additional value in the cardiac population. For cranial ultrasound, only one preoperative study was performed showing no predictive value for the later outcome. However median age at examination was 3 days of life and further studies are lacking.29
There were few centers performing MRI with sedation or general anesthesia as opposed to natural sleep. However, sedation and general anesthesia are associated with additional risks for patients and require further complex logistics and personnel resources. Thus institutional neuroimaging protocols that require anesthesia might pose a significant barrier to using MRI more frequently and on a clinical routine basis. However, protocols for performing neonatal MRI in natural sleep have been tested and validated.30,31 Furthermore, a number of studies at different sites have shown that MRI in natural sleep is feasible and can yield images of high quality in neonates with CHD.14,32,33 Exchanging experience in performing MRI in natural sleep among European pediatric heart centers could increase the use of perioperative MRI and help leverage this tool for the detection of brain injury in the vulnerable population of neonates with CHD.
In neonatal at-risk populations, a standardized neonatal neurological examination or assessment of General Movements has been shown to be a key tool in the early identification and prediction of motor impairments such as cerebral palsy.34 For the CHD population, studies have shown that standardized assessments such as the Neonatal Intensive Care Unit Network Neurobehavioral Scale,35,36 the Einstein Neonatal Neurobehavioral Assessment Scale37,38 or a General Movements assessment39 can reveal neurological and neurobehavioral abnormalities that are already evident in the preoperative and postoperative neonatal period and are associated with brain volumetric measurements on MRI.38 Furthermore, the General Movements assessment has been shown to have a good predictive value for later cognitive and neuromotor outcome.40,41 Thus, performing a standardized neurological assessment before hospital discharge is a crucial prerequisite to counsel parents, implement early support, and tailor follow-up and should be standard of care in all centers caring for neonates with complex CHD. However, among the responding centers across Europe, a postoperative neurological examination has been reported to be performed in only 20% of infants after cardiopulmonary bypass surgery, possibly leading to many missed opportunities of implementing early support for the child and their family. This current practice is in contrast to the recently published recommendations from the Cardiac Neurodevelopmental Outcome Collaborative on neurodevelopmental evaluation strategies for young children with CHD, which suggests performing a neurobehavioral consultation prior to hospital discharge in all infants with CHD.19 In line with other current recommendations19,20 the importance of neurodevelopmental follow-up in this vulnerable population has been recognized across Europe, with 2/3 of responding centers having already implemented or are planning to implement a structured follow-up program. However, outcome data collection is often not performed uniformly or systematically, which is a requirement for quality control and comparison across centers. Accordingly, the majority of participating centers have formulated the wish to join a European CHD outcome register. Furthermore, an adaptation of follow-up guidelines to European or even country-specific health care characteristics might be necessary to ensure broad and comprehensive implementation of structured follow-up programs, as a recent Canadian analysis demonstrated.42 To further investigate this, an in-depth evaluation of center-specific follow-up programs in European pediatric heart centers is warranted in future studies.
Limitation
This study has several limitations. As there was no standardized questionnaire available, we used a set of questions that were selected based on expert opinions from the European ABC Consortium. The questionnaire was not formally validated within an independent test set. Although we tried to reach out to all European heart centers by distributing our questionnaire via multiple channels, we were only able to cover a fraction of all centers caring for neonates with complex CHD. We found a dense and satisfiable response rate in central Europe, whereas many other (e.g., Scandinavian or eastern European) countries were missed. Considering that there are 32 national delegates in the AEPC, our sample is likely only representative of a small fraction of European centers and potentially overestimates the use of perioperative neuromonitoring tools and implementation of follow-up in Europe. The completion rate of the questionnaire relative to started questionnaires by clicks on the survey link was relatively low. However, sending out impersonalized links did not allow us to filter out multiple clicks on the link by the same respondents.
Conclusion
There is large heterogeneity in perioperative neuromonitoring and neuroimaging practice in centers across Europe. Despite evidence for the value of aEEG, MRI, and clinical examination in detecting and predicting adverse neurological outcomes in CHD a consistent and routine implementation in pediatric heart centers across Europe is lacking. The need for neurodevelopmental follow-up has been widely recognized. But programs could benefit from standardization and systematic recording by creating a European outcome register. This survey provides the basis for the development of a much-needed expert panel recommendation using for example a Delphi method, which may function as a controlled debate between a group of experts moving toward consensus.43 A clear evidence-based practice recommendation could foster collaboration across European pediatric heart centers and is urgently needed to support centers in the implementation of state-of-the-art neuromonitoring, neuroimaging, and follow-up to optimize care and thereby improve neurodevelopmental outcomes in infants with complex CHD.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Dolk, H., Loane, M. & Garne, E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 123, 841–849 (2011).
van der Bom, T. et al. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 8, 50–60 (2011).
Marino, B. S. et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a Scientific Statement from the American Heart Association. Circulation 126, 1143–1172 (2012).
Latal, B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin. Perinatol. 43, 173–185 (2016).
Marelli, A., Miller, S. P., Marino, B. S., Jefferson, A. L. & Newburger, J. W. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation 133, 1951–1962 (2016).
Miller, S. P. et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 357, 1928–1938 (2007).
Dimitropoulos, A. et al. Brain injury and development in newborns with critical congenital heart disease. Neurology 81, 241–248 (2013).
Peyvandi, S. et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 155, 291–300 e293 (2018).
Claessens, N. H. P. et al. Brain injury in infants with critical congenital heart disease: insights from two clinical cohorts with different practice approaches. J. Pediatr. 215, 75.e2–82.e2. (2019).
Feldmann, M. et al. Delayed maturation of the structural brain connectome in neonates with congenital heart disease. Brain Commun. 2, fcaa209 (2020).
Meuwly, E. et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci. Rep. 9, 10885 (2019).
Claessens, N. H. et al. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr. Res. 80, 668–674 (2016).
Claessens, N. H. P. et al. Brain microstructural development in neonates with critical congenital heart disease: an atlas-based diffusion tensor imaging study. Neuroimage Clin. 21, 101672 (2019).
Bertholdt, S. et al. Cerebral lesions on magnetic resonance imaging correlate with preoperative neurological status in neonates undergoing cardiopulmonary bypass surgery. Eur. J. Cardiothorac. Surg. 45, 625–632 (2014).
Claessens, N. H. P. et al. Amplitude-integrated electroencephalography for early recognition of brain injury in neonates with critical congenital heart disease. J. Pediatr. 202, 199.e1–205.e1 (2018).
Latal, B. et al. Postoperative amplitude-integrated electroencephalography predicts four-year neurodevelopmental outcome in children with complex congenital heart disease. J. Pediatr. 178, 55–60 e51 (2016).
Zaleski, K. L. & Kussman, B. D. Near-infrared spectroscopy in pediatric congenital heart disease. J. Cardiothorac. Vasc. Anesth. 34, 489–500 (2020).
Peyvandi, S. et al. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J. Am. Coll. Cardiol. 71, 1986–1996 (2018).
Ware, J. et al. Neurodevelopmental evaluation strategies for children with congenital heart disease aged birth through 5 years: recommendations from the Cardiac Neurodevelopmental Outcome Collaborative. Cardiol. Young. 30, 1609–1622 (2020).
Ilardi, D. et al. Neurodevelopmental evaluation for school-age children with congenital heart disease: Recommendations from the Cardiac Neurodevelopmental Outcome Collaborative. Cardiol. Young. 30, 1623–1636 (2020).
Stegeman, R. et al. A uniform description of perioperative brain MRI findings in infants with severe congenital heart disease: results of a European Collaboration. AJNR Am. J. Neuroradiol. 42, 2034–2039 (2021).
Akintuerk, H. et al. Stenting of the arterial duct and banding of the pulmonary arteries. Circulation 105, 1099–1103 (2002).
Claessens, N. H. P. et al. Postoperative cerebral oxygenation was not associated with new brain injury in infants with congenital heart disease. J. Thorac. Cardiovasc Surg. 158, 867–877.e861 (2019).
Shellhaas, R. A. et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J. Clin. Neurophysiol. 28, 611–617 (2011).
Mulkey, S. B. et al. Amplitude-integrated EEG in newborns with critical congenital heart disease predicts preoperative brain magnetic resonance imaging findings. Pediatr. Neurol. 52, 599–605 (2015).
Gunn, J. K., Beca, J., Hunt, R. W., Olischar, M. & Shekerdemian, L. S. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. 38, 1539–1547 (2012).
Claessens, N. H. P. et al. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev. Med. Child Neurol. 60, 1052–1058 (2018).
Mahle, W. T. et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 106, I109–I114 (2002).
Latal, B. et al. Can preoperative cranial ultrasound predict early neurodevelopmental outcome in infants with congenital heart disease? Dev. Med. Child Neurol. 57, 639–644 (2015).
Mathur, A. M., Neil, J. J., McKinstry, R. C. & Inder, T. E. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr. Radiol. 38, 260–264 (2008).
Hughes, E. J. et al. A dedicated neonatal brain imaging system. Magn. Reson. Med. 78, 794–804 (2017).
Kelly, C. J. et al. Neuroimaging findings in newborns with congenital heart disease prior to surgery: an observational study. Arch. Dis. Child. 104, 1042–1048 (2019).
Lim, J. M. et al. Associations between age at arterial switch operation, brain growth, and development in infants with transposition of the great arteries. Circulation 139, 2728–2738 (2019).
Novak, I. et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 171, 897–907 (2017).
Massaro, A. N. et al. Neurobehavioral abnormalities in newborns with congenital heart disease requiring open-heart surgery. J. Pediatr. 158, 678.e2–681.e2 (2011).
Hogan, W. J. et al. Neurobehavioral evaluation of neonates with congenital heart disease: a cohort study. Dev. Med. Child Neurol. 60, 1225–1231 (2018).
Limperopoulos, C. et al. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J. Pediatr. 137, 638–645 (2000).
Owen, M. et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J. Pediatr. 164, 1121.e1–1127.e1 (2014).
Huisenga, D. C., Van Bergen, A. H., Sweeney, J. K., Wu, Y. C. & Hadders-Algra, M. The quality of general movements in infants with complex congenital heart disease undergoing surgery in the neonatal period. Early Hum. Dev. 151, 105167 (2020).
Groen, S. E., De Blécourt, A. C., Postema, K. & Hadders‐Algra, M. General movements in early infancy predict neuromotor development at 9 to 12 years of age. Dev. Med. Child Neurol. 47, 731–738 (2005).
Bruggink, J. L., Van Braeckel, K. N. & Bos, A. F. The early motor repertoire of children born preterm is associated with intelligence at school age. Pediatrics 125, e1356–e1363 (2010).
Bolduc, M.-E., Rennick, J. E., Gagnon, I., Majnemer, A. & Brossard-Racine, M. Canadian developmental follow-up practices in children with congenital heart defects: a national environmental scan. CJC Pediatr. Congenit. Heart Dis. 1, 3–10 (2022).
Linstone, H. & Turoff, M. The Delphi Method: Techniques and Applications (Addison Wesley Publishing, 1975).
Acknowledgements
We would like to thank all members of the European Association Brain in Congenital Heart Disease (European ABC) for their contribution to this work: R. Stegeman, N.H.P. Claessens, M. Nijman, F. Haas, R. Kottke, J. Simpson, A.F. Bonthrone, C.J. Kelly, S. Arulkumaran, M.A. Rutherford, D. Cromb, M. Haak, C. Steger, A. De Silvestro, C. Austin, F. Groenendaal, J. Nijman.
Funding
This research was funded by a Consolidator Grant of the European Society of Paediatric Research (ESPR). Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Contributions
M.F. contributed to the conception and design, acquisition of data and data analysis, and drafted and revised the manuscript. C.H., L.d.V., K.P., T.L., N.J.G.J., J.M.P.J.B., W.K., M.B., S.C., and B.R. contributed to the conception and design, helped to interpret the data, and revised the manuscript. V.D. drafted and revised the manuscript and helped interpret and analyze the data. B.L. contributed to the conception and design, acquisition of data and data analysis, and drafting and revising of the manuscript. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feldmann, M., Hagmann, C., de Vries, L. et al. Neuromonitoring, neuroimaging, and neurodevelopmental follow-up practices in neonatal congenital heart disease: a European survey. Pediatr Res 93, 168–175 (2023). https://doi.org/10.1038/s41390-022-02063-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02063-2
This article is cited by
-
Perioperative Neuromonitoring in Children with Congenital Heart Disease
Neurocritical Care (2024)