Abstract
Background
Attention deficit-hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders in children; however, studies delineating the association between ADHD and central precocious puberty are limited. This study aimed to understand whether children with ADHD are at a higher risk of central precocious puberty.
Methods
This population-based retrospective cohort study was conducted using the National Health Insurance Research Database of Taiwan to investigate the association between ADHD and the incidence of central precocious puberty between 2000–2015. We identified ADHD individuals treated with methylphenidate, atomoxetine or not. The control cohort consisted of individuals without ADHD. The outcome measure was central precocious puberty diagnosis.
Results
Among 290,148 children (mean age: 5.83 years), central precocious puberty incidence was 4.24 and 1.95 per 105 person-years in the ADHD and control groups, respectively. Children with ADHD treated with medication had a higher risk than those without ADHD. However, medication use did not affect the incidence of central precocious puberty among children with ADHD.
Conclusion
This study showed an association between ADHD and a higher risk of central precocious puberty. Early referral of children with ADHD to a pediatric endocrinologist for evaluation may facilitate correct diagnoses and early interventions.
Impact
-
ADHD is associated with a higher risk of central precocious puberty.
-
This study provides relevant findings, as it is the first nationwide, population-based cohort study to investigate the association between ADHD and the risk of central precocious puberty with a 15-year follow-up.
-
Early referral of children with ADHD to a pediatric endocrinologist for the evaluation of suspected precocious puberty could facilitate correct diagnosis.
-
Early intervention treatment with gonadotropin-releasing hormone agonist might improve final height in children with central precocious puberty.
Similar content being viewed by others
Introduction
Attention deficit-hyperactivity disorder (ADHD) is a common chronic neurobehavioral disorder that occurs during childhood and is characterized by disruptive inattention, hyperactivity, and impulsivity. Children with ADHD usually experience academic underachievement, problems with interpersonal relationships with family members and friends, and low self-esteem. ADHD also often coincides with other emotional, behavioral, language, and learning disorders.1 In 2015, the estimated worldwide prevalence of ADHD in children and adolescents was 7.2% (95% confidence interval [CI]: 6.7–7.8).2 To date, there is no single identified cause of ADHD; however, inherited and non-inherited factors independently contribute to disease manifestation.3,4 Several risk factors for ADHD have been identified, including family history of ADHD, history of adoption, maternal smoking, alcohol use during pregnancy, premature birth, low birth weight, intrauterine growth restriction, history of brain injury, developmental delay, and genetic risk variants.5,6,7 In addition, dysregulation of neurotransmitter pathways, such as central dopaminergic and noradrenergic pathways, within the brain is presumed to be the pathophysiological basis for ADHD.8 Management of ADHD varies by age; the American Academy of Pediatrics recommends behavioral therapy and medication, emphasizing differences in the treatment of preschool, school-age, and adolescent patients.3,9 Behavioral therapy is the first-line treatment for children aged 4–5 years and could improve ADHD symptoms in both children and adolescents.10 Food and Drug Administration-approved medications for ADHD in children aged >6 years and adolescents include stimulants (i.e., amphetamines, short-acting and long-acting methylphenidate), serotonin–noradrenaline reuptake inhibitors (i.e., atomoxetine), clonidine, and guanfacine. The prevalence of medication use in children with ADHD increased from 2001 to 2015, and stimulants are widely used as the first-line medication for ADHD. Medications used to treat ADHD are effective in reducing inattention, hyperactivity, and impulsivity and might improve academic achievement while being well-tolerated long-term (up to 24 months).11,12 Methylphenidate has been used to treat ADHD and is generally considered to be safe.13 Children with ADHD need parents, teachers, psychiatrists, and pediatricians to develop a treatment strategy;8,14 however, understanding of ADHD remains limited.
Precocious puberty is traditionally defined as the appearance of secondary sexual characteristics before 8 and 9 years of age in girls and boys, respectively.15 This can lead to increased growth rate, sexual development, and more rapid skeletal maturation. Precocious puberty incidence varies among ethnicities; it is reported to occur in 0.2% of girls and <0.05% of boys in Denmark16 and in 5.66 per million person-years in Spain.17 In South Korea, precocious puberty is found in 122.8 per 100,000 children.18 The cause of central precocious puberty most commonly remains idiopathic.19 Risk factors for central precocious puberty also include congenital or acquired central nervous system (CNS) disorders and genetic causes.20 Central precocious puberty may contribute to the dysregulation of the CNS. Early activation of the hypothalamic–pituitary–gonadal axis leads to increased gonadotropin-releasing hormone secretion and is followed by the release of gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone, thus leading to an increase in serum gonadal sex hormone level, which ultimately leads to physical manifestations of puberty.21 To date, the mechanism of CNS regulation remains unclear. Pubertal LH response (LH peak >5 IU/L) has been used as a criterion for central precocious puberty.22
To our knowledge, several children with ADHD undergoing regular treatment were followed up in the hospital and developed central precocious puberty. These patients were referred to, and had their diagnosis confirmed by, a pediatric endocrinologist. However, the association between ADHD and central precocious puberty remains unknown. Here, we hypothesized that children with ADHD have a higher risk of developing central precocious puberty. Hence, we performed this nationwide, population-based, retrospective cohort study to evaluate central precocious puberty risk in children with ADHD in Taiwan. This study demonstrated the association between ADHD and a higher risk of central precocious puberty.
Methods
Data sources
We explored the association between ADHD and central precocious puberty incidence over a 15-year period using data from the Taiwan National Health Insurance Research Database (NHIRD). We extracted documents involving ADHD from the outpatient and hospitalization records between 2000 and 2015 in the Taiwan Longitudinal Health Insurance Database. Healthcare in Taiwan is administered by the National Health Insurance Program (NHIP), initiated in 1995. It consists of agreements with 97% of the health care service providers and covers >99% of the total population in Taiwan—approximately 23 million recipients in June 2009.23 Although Taiwan is an immigrant and hybrid society, this study population is ethnically homogenous; thus, the findings may not be generalizable. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), was applied by the NHIRD to track diagnoses. Literature reviews acknowledged the advantages, limitations, and details of the NHIRD.24
All ADHD diagnoses were confirmed by pediatricians, general practitioners, or psychiatrists in accordance with the clinical findings. Precocious puberty diagnoses were made by pediatric endocrinologists and defined as the onset of secondary sexual characteristics before ages 8 and 9 years and in girls and boys, respectively. Consequently, we utilized the NHIRD documents to analyze the connection between ADHD and precocious puberty incidence. This study was approved by the Institutional Review Board of Tri-Service General Hospital (TSGHIRB No. C202105013), Taiwan.
Study design and populations
This study used a retrospective matched cohort design. We enrolled girls aged <8 years and boys aged <9 years diagnosed with ADHD (at least twice) in an outpatient department (OPD) or a hospital between January 1, 2000, and December 31, 2015. Overall, 217,611 children without ADHD, matched for age, sex, and index year, were assigned to the control group. The relationship between pharmacological treatments for ADHD (including short- and long-acting methylphenidate and atomoxetine) and central precocious puberty were also analyzed. We excluded individuals who had precocious puberty or ADHD before tracking, CNS infection, CNS abnormality, any malignancy, primary hypothyroidism, diabetes mellitus, disorders of the parathyroid gland, acromegaly and gigantism, pituitary disorders, Cushing’s syndrome, hyperaldosteronism, or disorders of the adrenal glands; boys aged >9 years; and girls aged >8 years. We included children with central precocious puberty diagnoses but excluded individuals who had peripheral precocious puberty diagnoses. However, benign or nonprogressive pubertal variants (e.g., premature thelarche, premature adrenarche) and central precocious puberty could not be distinguished by ICD-9 codes in our study due to limitations of the NHIRD. Children with BMI ≥ 95th percentile for age and sex were diagnosed with obesity. Detailed study population characteristics, events, exclusion criteria, and the corresponding ICD_9 codes are listed in \ S1.
Covariates
We analyzed many covariates including age, sex, obesity, region of dwelling (Northern, Central, Southern, and Eastern Taiwan), degree of urbanization from level 1 to 4, category of the hospital, including medical centers, regional hospital, or local hospital, and category of insurance premium: less than 18,000, 18,000–34,999, or more than 35,000 in New Taiwan Dollars [NT$]. We defined the urbanization level based on population size and various indicators of development. A region with a specific designation of cultural, political, economic, and metropolitan development and a population of more than 1,250,000 citizens was in urbanization level 1. A region with 500,000–1,249,999 citizens that plays a crucial role in the culture, political system, and economy was in urbanization level 2. Regions with populations of 149,999–499,999 and those with less than 149,999 citizens were in urbanization levels 3 and 4, respectively.25 Because we could not retrieve the birthweight of children based on the limitations of the NHIRD, birthweight was not included as a covariate in this study.
Exposure and study outcomes
We followed up all candidates from the start of follow-up until the diagnosis of central precocious puberty, cancellation from the NHIP, or until the end of the study period. Sensitivity analyses were conducted by excluding the first year (2000–2001) and the first 5 years (2000–2005) to avoid any potential confounding factors (for example, cases in which the participants had ADHD or precocious puberty before the year 2000 but were undiagnosed). The contribution of ADHD medications was also analyzed, and we determined whether pharmaceutical agents increased or decreased the risk of central precocious puberty.
Statistical analysis
All statistical analyses were carried out using IBM SPSS Statistics version 22.0 for Windows (SPSS Inc., Chicago, IL). Chi-squared and two-tailed Student’s t tests were applied to compare continuous and categorical variables between ADHD and non-ADHD groups, respectively; Fisher’s exact test was used to determine nonrandom associations between two categorical variables, and Cox regression analysis was applied to investigate the risk of precocious puberty. Hazard ratios (HRs) with 95% CIs were used to present results. The Kaplan–Meier method and the log-rank test were applied to evaluate the differences in the risk of developing precocious puberty between the ADHD and non-ADHD groups. P < 0.05 in two-tailed tests were considered statistically significant.
Results
Baseline characteristics of study participants
In total, 290,148 children (boys aged <9 years and girls aged <8 years) were enrolled, and 72,537 were identified to have ADHD, which was coded at least twice at an OPD or in a hospital. The other 217,611 children were assigned as controls and were matched for age, sex, and index year (Fig. 1). The mean age of the cohort study was 5.8 ± 4.9 years, and boys accounted for 66.33%. No significant differences in terms of sex, age, or obesity were noted between the two groups. The distribution of insurance premium categories, location and urbanization level, and hospital care level of the enrolled participants differed between the two groups. The detailed characteristics are shown in Table S2. The mean age at baseline among children with ADHD and children without ADHD was 5.81 and 5.83, respectively. The mean age at the endpoint among children with ADHD and children without ADHD was 11.28 and 12.94, respectively. Among all participants, children with ADHD, and children without ADHD, the median years of follow-up and IQRs were 7.95, 3.30-10.77; 7.89, 3.25-10.56; and 7.97, 3.34-10.79; respectively. The detailed years of follow-up data are listed in Table S4.
Incidence rate of central precocious puberty and HR
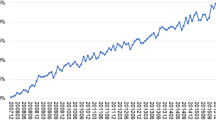
Central precocious puberty incidence was calculated as 4.24 per 105 person-years and 1.95 per 105 person-years in children with, and those without ADHD, respectively (p = 0.003; chi-square test; Table 2). Higher risks of central precocious puberty were noted in children with ADHD, with an adjusted HR of 1.51 (95% CI: 1.24–1.80, p = 0.003; Table 1). Girls had a higher risk of developing central precocious puberty than boys, with an adjusted HR of 1.76 (95% CI: 1.12–2.54, p < 0.001). Obesity status and children’s residence were not significantly correlated with central precocious puberty. However, the level of urbanization of residence had a significant impact. Children living in regions with urbanization levels 1 and 2 exhibited higher crude HRs for central precocious puberty (1.60 and 1.25, respectively), than those in the lowest level of urbanization. Children treated in medical centers and regional hospitals revealed higher crude HRs of central precocious puberty than those treated in local hospitals (1.57 and 1.27, respectively). Complete data are shown in Table 2. The 15-year cumulative incidence of central precocious puberty is significantly higher in children with ADHD than those without, as estimated by Kaplan–Meier analysis (log-rank test, p < 0.001; Fig. 2).
ADHD medication and risk of central precocious puberty
Three ADHD medications used in children and adolescents, including short-acting and long-acting methylphenidate and atomoxetine, were analyzed. In this cohort, 50.67% of children with ADHD were treated with one of the three medications, and 49.33% were not treated with any medication. The incidence of central precocious puberty in children with ADHD who were prescribed medication was 4.48 per 105 person-years, whereas that in children with ADHD without medication was 3.99 per 105 person-years (p = 0.512). The most frequently used ADHD medication was short-acting methylphenidate, accounting for 27.27% of children with ADHD. The incidence of central precocious puberty among children using short-acting and long-acting methylphenidate and atomoxetine was 5.20, 3.67, and 3.60 per 105 person-years, respectively. However, no significant difference was found between children with ADHD receiving medications and those with ADHD not receiving treatment (p = 0.51). Children with ADHD being treated with medications had a higher risk than those without ADHD (HR: 1.59, 95% CI: 1.30–1.89, p = 0.001).
Sensitivity analysis
Children with ADHD had an increased risk of central precocious puberty. These associations remained significant after excluding the information for participants in 2000–2001 and in 2000–2005, respectively. The corrected incidence rates of precocious puberty after excluding the first year (2000–2001) and the first 5 years (2000–2005) in children with ADHD were 4.23 and 4.11 per 105 person-years, respectively, whereas those in children without ADHD were 2.08 and 2.03 per 105 person-years, respectively. The adjusted HRs after excluding the first year (2000–2001) and the first 5 years (2000–2005) were 1.41 and 1.41, respectively (p = 0.007 and 0.009, respectively; Table 3).
Discussion
Our results revealed that children with ADHD have a higher risk of central precocious puberty, with an HR of 1.51. Even after excluding the data from 2000–2001 and 2000–2005, children with ADHD still had a higher risk, with an HR of 1.41 (Table 3). The risk of central precocious puberty was higher in girls. We also found that children living in more urbanized areas had higher risks of developing central precocious puberty. Most importantly, taking ADHD medication had no effect on the risk of precocious puberty. There is little evidence in the literature regarding an association between ADHD and central precocious puberty. A review study assumed that sex hormones may impact neuronal circuits that affect emotional and cognitive responses.26 Exploratory research found evidence for the association between precocious puberty, inattention, and risk-taking behavior.27 As far as we know, this is the first nationwide, population-based cohort study to investigate the association between ADHD and the risk of central precocious puberty with a 15-year follow-up.
ADHD has been investigated mainly in field trials with children aged between 5–12 years. The prevalence of ADHD was reported to be lower in Taiwan (as low as 4.2%) than the worldwide prevalence.2,28,29 ADHD is more common in boys than girls, with a sex ratio ranging from 2:1 to 10:1.30 In this study, male patients accounted for 66.33% of the study population, and the mean age during enrollment and at the end of the study in the ADHD group was 5.81 and 9.73 years old, respectively. The patient characteristics of the study cohort in the ADHD group were similar to those of other studies. Girls were much more likely to develop central precocious puberty (incidence in girls: 20 per 10,000; incidence in boys: <5 per 10,000), as reported by a population-based study of data from Danish national registries between 1993 and 2001.16 In the present study, girls accounted for 93.0% of children with central precocious puberty (both in the ADHD and control groups). The sex distribution in this study was similar to that of other studies.
In both ADHD and control groups, females were at a higher risk of central precocious puberty than males, with an adjusted HR of 1.76 (95% CI: 1.12–2.54, p < 0.001). Considering that most individuals with precocious puberty were females, we analyzed the impact of ADHD on central precocious puberty risk in both sexes to avoid any confounding effect. The male incidence rate of central precocious puberty in the ADHD and control groups were 0.43 and 0.22 per 105 patient-years, respectively (HR: 1.37, 95% CI: 1.13–1.64, p = 0.006). Conversely, the female incidence rate of central precocious puberty in the ADHD and control groups were 11.38 and 5.17 per 105 patient-years, respectively (HR: 1.53, 95% CI: 1.25–1.82, p < 0.001). Both female and male patients with ADHD had an increased risk of central precocious puberty.
Other covariates included obesity, insurance premium, which reflects family income, urbanization level of residence, and level of hospital care. As previously mentioned, children living in more urbanized areas and receiving higher levels of hospital care, where most pediatric endocrinologists work, had a greater risk of developing central precocious puberty. However, children with ADHD had larger proportions of higher urbanization level of residence and level of hospital care compared to children without ADHD. This may lead to an overestimation of the association. We separately analyzed the association between ADHD and central precocious puberty risk as a function of insurance premium, urbanization level, and level of hospital care. We found that children with ADHD in lower insurance premium categories, higher urbanization levels, and all hospital care levels have a higher risk of central precocious puberty. (Table S3). Obesity was not a significant risk factor for precocious puberty in either group. However, the impact of obesity, or the lack thereof, could not be analyzed. Hwang et al. reported that infants born small for gestational age (SGA) tend to have short stature, neurocognitive dysfunction, and metabolic syndrome. These children are also prone to precocious puberty, premature adrenarche, and faster progression of puberty.31 Verkauskiene et al. revealed that puberty starts within the normal range of age in SGA children, but that onset is earlier relative to appropriate for gestational age children. As a result, if children with ADHD have a higher percentage of low birth weight compared to children without ADHD, it might lead to a higher risk of precocious puberty.32
Although the etiology of ADHD and central precocious puberty remain unclear, both are thought to be associated with CNS dysregulation.33,34 The key role of the dopamine system in ADHD has been suggested by pharmacology and genetic studies of animal models.35,36,37 Regarding central precocious puberty, activation of the hypothalamic–pituitary–gonadal axis was associated with increased growth rate, onset of sexual development, and more rapid skeletal maturation.38 We observed this association between ADHD and central precocious puberty in this population-based nationwide cohort study. Further prospective registration databases or mechanistic studies are required to provide more information.
Methylphenidate, a stimulant used to treat ADHD, may cause adverse effects on the maturation and functionality of the female reproductive system, as revealed by previous animal studies.39 Another study described the relationship between methylphenidate administration and the central precocious puberty hypothesis, which suggests that the excitatory effect of dopamine- and noradrenaline-induced gonadotropin-releasing hormone release, combined with the inhibition of gonadotropin-releasing hormone receptor expression, suppresses the effect of prolactin on the hypothalamic–pituitary–gonadal axis.40 In this study, ~50% of children with ADHD were treated with methylphenidate (short- or long-acting) or atomoxetine. The most common medication used was short-acting methylphenidate. The risk of central precocious puberty was not statistically different for different pharmacological treatments of ADHD in children. Furthermore, medication use did not increase the risk of central precocious puberty in children with ADHD.
As children with central precocious puberty are diagnosed by pediatric endocrinologists, long-term observation is important before treatment is considered. We could predict the child’s adult height by measuring bone maturation. According to a recent literature review, the final adult height of children with central precocious puberty was below the normal mean with standard deviation scores of −2.5/−3.0 in boys and –1 in girls.41 Treatment with gonadotropin-releasing hormone agonist improved final height in children with central precocious puberty.42 The present study, with a retrospective matched cohort design, used the NHIRD, which restricts personal information; therefore; we could not retrieve the heights of children with ADHD who had central precocious puberty. Further, we could not determine what kind of treatment children with ADHD and central precocious puberty received. However, the relationship between treatment for children with central precocious puberty and ADHD is interesting and deserves further study in the future.
This study also had several limitations. First, the database did not provide actual medical records, limiting a thorough understanding of whether the symptoms met the diagnostic criteria for ADHD and precluding the confirmation of the signs of puberty. Second, the diagnoses in the database were made by different specialists; therefore, the standard for diagnosis may have varied. Third, benign or nonprogressive pubertal variants such as premature thelarche and premature adrenarche were not differentiated from central precocious puberty. This could have inflated the incidence in our study. Fourth, our study did not explore whether the dosages and time frames of ADHD medication were associated with central precocious puberty. Fifth, the etiologies of both ADHD and central precocious puberty are complex and are affected by genetics, history of adoption, family history, exposure to environmental toxins and pollutants, and even the extensive use of plastics and preservatives, which are factors that could not be identified in this cohort. Finally, selection bias cannot be excluded due to the retrospective nature of the study. Children with ADHD who already had precocious puberty before tracking were excluded. Thus, the study could not assess both directions of association, that is, children who were diagnosed with precocious puberty first and then with ADHD (either in the control group or children who were excluded from the study entirely). This may have led to an underestimation of the association, and future randomized controlled trials are being considered. Studies on ADHD in the general population are limited, and the prevalence of ADHD may have been underestimated in Taiwan. In the future, additional studies should investigate the relationship of ADHD with central precocious puberty.
We evaluated the association between central precocious puberty and ADHD in the general pediatric population in Taiwan by analyzing representative population-based data. Our findings revealed an association between ADHD and an increased risk of central precocious puberty. Thus, all caregivers of children with ADHD, including relatives, schoolteachers, nurses, pediatricians, and psychiatrists, should be aware that children with ADHD might have a higher risk of developing central precocious puberty. Early referral of children with ADHD to a pediatric endocrinologist for the evaluation of suspected precocious puberty could facilitate correct diagnosis. Early intervention treatment with gonadotropin-releasing hormone agonist might improve final height in children with central precocious puberty.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Change history
15 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41390-023-02692-1
References
Thapar, A. & Cooper, M. Attention deficit hyperactivity disorder. Lancet 387, 1240–1250 (2016).
Thomas, R., Sanders, S., Doust, J., Beller, E. & Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135, e994–e1001 (2015).
Feldman, H. M. & Reiff, M. I. Clinical Practice. Attention deficit-hyperactivity disorder in children and adolescents. N. Engl. J. Med. 370, 838–846 (2014).
Christakis, D. A., Zimmerman, F. J., DiGiuseppe, D. L. & McCarty, C. A. Early television exposure and subsequent attentional problems in children. Pediatrics 113, 708–713 (2004).
Momany, A. M., Kamradt, J. M. & Nikolas, M. A. A meta-analysis of the association between birth weight and attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 46, 1409–1426 (2018).
Adeyemo, B. O. et al. Mild traumatic brain injury and Adhd: a systematic review of the literature and meta-analysis. J. Atten. Disord. 18, 576–584 (2014).
Grimm, O., Kranz, T. M. & Reif, A. Genetics of Adhd: what should the clinician know. Curr. Psychiatry Rep. 22, 18 (2020).
Sharma, A. & Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (Adhd). Ann. Pharmacother. 48, 209–225 (2014).
Magnus, W., Nazir, S., Anilkumar, A. C. & Shaban, K. in Statpearls (StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC., 2021).
Sonuga-Barke, E. J. et al. Nonpharmacological interventions for Adhd: systematic review and meta-analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. Am. J. Psychiatry 170, 275–289 (2013).
Cortese, S. Pharmacologic treatment of attention deficit–hyperactivity disorder. N. Engl. J. Med. 383, 1050–1056 (2020).
Kemper, A. R. et al. in Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents (Agency for Healthcare Research and Quality (US), 2018).
Storebo, O. J. et al. Methylphenidate for Attention Deficit Hyperactivity Disorder (Adhd) in children and adolescents - assessment of adverse events in non-randomised studies. Cochrane Database Syst. Rev. 5, CD012069 (2018).
Wolraich, M. et al. Adhd: Clinical Practice Guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128, 1007–1022 (2011).
Boepple Pa, Crowley W. F. Jr. Precocious Puberty. In: Reproductive Endocrinology, Surgery, and Technology, Adashi Ey, Rock Ja, Rosenwaks Z. (Eds), Lippincott-Raven, Philadelphia 1996. Vol 1, P. 989.
Teilmann, G., Pedersen, C. B., Jensen, T. K., Skakkebaek, N. E. & Juul, A. Prevalence and Incidence of Precocious Pubertal Development in Denmark: An Epidemiologic Study Based on National Registries. Pediatrics 116, 1323–1328 (2005).
Soriano-Guillen, L. et al. Central Precocious Puberty in Children Living in Spain: Incidence, Prevalence, and Influence of Adoption and Immigration. J. Clin. Endocrinol. Metab. 95, 4305–4313 (2010).
Kim, Y. J. et al. Incidence and Prevalence of Central Precocious Puberty in Korea: An Epidemiologic Study Based on a National Database. J. Pediatr. 208, 221–228 (2019).
Klein, D. A., Emerick, J. E., Sylvester, J. E. & Vogt, K. S. Disorders of Puberty: an approach to diagnosis and management. Am. Fam. Physician 96, 590–599 (2017).
Cantas-Orsdemir, S. & Eugster, E. A. Update on Central Precocious Puberty: From Etiologies to Outcomes. Expert Rev. Endocrinol. Metab. 14, 123–130 (2019).
Fuqua, J. S. Treatment and Outcomes of Precocious Puberty: An Update. J. Clin. Endocrinol. Metab. 98, 2198–2207 (2013).
Kliegman, R. M. Nelson Textbook of Pediatrics; Elsevier: Philadelphia, Pa, USA, 2020; P. 11340.
Ho Chan, W. S. Taiwan’s Healthcare Report 2010. EPMA J. 1, 563–585 (2010).
Hsieh, C. Y. et al. Taiwan’s National Health Insurance Research Database: past and future. Clin. Epidemiol. 11, 349–358 (2019).
Chang, C. Y. et al. Increased risk of major depression in the three years following a Femoral Neck Fracture-a National Population-Based Follow-up Study. PLoS ONE 9, e89867 (2014).
Haimov-Kochman, R. & Berger, I. Cognitive functions of regularly cycling women may differ throughout the month, depending on sex hormone status; a possible explanation to conflicting results of studies of Adhd in females. Front. Hum. Neurosci. 8, 191 (2014).
D. Ostojic, C. M. Association between Pubertal Onset and Symptoms of Adhd in Female University Students. J. Atten. Disord. 20, 782–791 (2016).
Wang, L. J. et al. Prevalence rates of youths diagnosed with and medicated for Adhd in a Nationwide Survey in Taiwan from 2000 to 2011. Epidemiol. Psychiatr. Sci. 26, 624–634 (2017).
Liu, A., Xu, Y., Yan, Q. & Tong, L. The prevalence of attention deficit/hyperactivity disorder among Chinese Children and Adolescents. Sci. Rep. 8, 11169 (2018).
Mowlem, F. D. et al. Sex differences in predicting Adhd clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry 28, 481–489 (2019).
Hwang, I. T. Long-term care, from neonatal period to adulthood, of children born small for gestational age. Clin. Pediatr. Endocrinol. 28, 97–103 (2019).
Verkauskiene, R., Petraitiene, I. & Albertsson Wikland, K. Puberty in children born small for gestational age. Horm. Res. Paediatr. 80, 69–77 (2013).
Genro, J. P., Kieling, C., Rohde, L. A. & Hutz, M. H. Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev. Neurother. 10, 587–601 (2010).
Chirico, V. et al. Central Precocious Puberty: from physiopathological mechanisms to treatment. J. Biol. Regul. Homeost. Agents 28, 367–375 (2014).
van der Kooij, M. A. & Glennon, J. C. Animal Models Concerning the Role of Dopamine in attention-deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 31, 597–618 (2007).
Engert, V. & Pruessner, J. C. Dopaminergic and Noradrenergic Contributions to Functionality in Adhd: the role of methylphenidate. Curr. Neuropharmacol. 6, 322–328 (2008).
Gizer, I. R., Ficks, C. & Waldman, I. D. Candidate Gene Studies of Adhd: a meta-analytic review. Hum. Genet. 126, 51–90 (2009).
Latronico, A. C., Brito, V. N. & Carel, J. C. Causes, Diagnosis, and Treatment of Central Precocious Puberty. Lancet Diabetes Endocrinol. 4, 265–274 (2016).
Chatterjee-Chakrabarty, S., Miller, B. T., Collins, T. J. & Nagamani, M. Adverse effects of methylphenidate on the reproductive axis of adolescent female rats. Fertil. Steril. 84(Suppl 2), 1131–1138 (2005).
Ergur, A. T., Gul, H. & Gul, A. Methylphenidate and Central Precocious Puberty: a probable side effect among seven children with the attention deficit hyperactivity disorder. Clin. Psychopharmacol. Neurosci. 17, 446–449 (2019).
Mucaria, C., Tyutyusheva, N., Baroncelli, G. I., Peroni, D. & Bertelloni, S. Central precocious puberty in boys and girls: similarities and differences. Sexes 2, 119–131 (2021).
Klein, K. O., Barnes, K. M., Jones, J. V., Feuillan, P. P. & Cutler, G. B. Jr. Increased final height in precocious puberty after long-term treatment with Lhrh Agonists: The National Institutes of Health Experience. J. Clin. Endocrinol. Metab. 86, 4711–4716 (2001).
Acknowledgements
We appreciate the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW), Taiwan for providing access to the National Health Insurance Research Database (NHIRD). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Funding
This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-B-111018, TSGH-E-110186, TSGH-E-111195), and the sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.S.W. and D.M.C. Methodology: D.S.W., S.J.C. Software: C.H.C. and W.C.C. Validation: D.S.W. and D.M.C. Formal analysis: C.H.C. and W.C.C. Investigation: W.F.H. and S.W.H. Resources: C.H.C. and W.C.C. Data curation: D.S.W. Writing–original draft preparation: L.F.P. and D.S.W. writing–review and editing: W.C.C. and D.M.C. Visualization: L.F.P. Supervision: D.M.C. Project administration: D.M.C. Funding acquisition: D.M.C.
Corresponding authors
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the affiliation 8 were incorrectly inserted.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pai, LF., Wang, DS., Hsu, WF. et al. New insights into precocious puberty and ADHD: a nationwide cohort study. Pediatr Res 92, 1787–1794 (2022). https://doi.org/10.1038/s41390-022-02028-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02028-5