Abstract
Background
Asthma is a chronic long-term inflammatory disease of the airways and is a major global health challenge. The primary aim of this study was to investigate the association between hypertensive disorders of pregnancy (HDP) and the risk of asthma at or before the age of seven years using the UK Millennium Cohort Study (MCS).
Methods
Overall, 18,552 families were recruited for wave one of the MCS when the child was 9 months old, and subsequently participated in waves two, three and four when they were three, five and seven years respectively. HDP were self-reported by mothers in wave one. The primary outcome was a parent-reported diagnosis of “ever asthma” at seven years, based on responses to a validated questionnaire.
Results
Following adjustment for a range of potential confounding factors, HDP was found to be associated with asthma in the offspring (adjusted odds ratio (AOR 1.35; 95% CI: 1.15–1.59)). A larger effect estimate was observed amongst children exposed to HDP and born preterm (AOR 1.81; 95% CI: 1.25–2.61) or small for gestational age (SGA) (AOR = 1.58; (95% CI: 1.15–2.18)).
Conclusions
In conclusion, children exposed to HDP may be at increased odds of asthma diagnosis by age seven, particularly if they were born preterm or SGA.
Impact
-
There is a paucity of data investigating the relationship between hypertensive disorders of pregnancy and childhood asthma, with recent studies showing conflicting results.
-
Our study investigated this relationship using a large cohort with ample information on a wide range of confounding factors.
-
Our study showed that individuals exposed to HDP may be at increased odds of asthma diagnosis by age seven years, particularly if they were also born SGA or preterm.
Similar content being viewed by others
Introduction
Asthma is a chronic long-term inflammatory disease of the airways and is a major global health challenge, with an estimated prevalence of one in five children in the UK.1,2 Children with asthma are more likely to be hospitalised, require more frequent healthcare visits, miss more day-care/school, and their parents are more likely to be absent from work as a result.3,4 In countries without universal healthcare, severe and uncontrolled asthma can cause a high financial burden for families.5 Recent evidence suggests the National Health Service in the UK spends in excess of £1.1 billion on asthma annually.6 The significant health and socioeconomic impacts of asthma have underscored the need to identify the contributing factors that increase the risk of an asthma diagnosis in childhood, to improve aetiological understanding, and to allow more targeted surveillance of those most at risk.
There is a growing body of evidence suggesting that exposure to an adverse intrauterine environment is involved in the aetiology of childhood respiratory conditions, including asthma. One such potential risk factor is fetal exposure to hypertensive disorders of pregnancy (HDP) which affect 10–15% of pregnancies.7 HDP is an umbrella term for several hypertensive conditions including gestational hypertension, chronic hypertension, pre-eclampsia and eclampsia.7 HDP have been reported to be associated with impaired lung function in infancy.8 However, the effect of HDP on lung function in later childhood is unclear, with studies showing conflicting results.
Pre-eclampsia,9,10,11 maternal essential hypertension12 and individual readings of high maternal blood pressure13 have been associated with increased risk of asthma in the offspring in the recent registry-based and cohort studies. However, other studies have contradicted these findings reporting no association between pre-eclampsia,14,15 gestational hypertension,16 or chronic hypertension17 with childhood asthma.
The inconsistencies in the current literature highlight the importance of studying the effect of HDP on the risk of asthma in the offspring in large cohorts which have ample information on a wide range of confounders and used validated tools to assess a diagnosis of asthma. Thus, the primary aim of this study was to explore the association between HDP and the development of asthma at or before the age of seven years using the UK population-based Millennium Cohort Study (MCS).
Methods
Study population
The Millennium Cohort Study (MCS) is a nationally representative sample drawn from all births in the UK between 2000–2002.18 Eligible children were identified using government child benefit records, a benefit with almost universal coverage.19 Surveys of the MCS have been conducted in several waves including age 9 months (wave 1), 3 years (wave 2), 5 years (wave 3) and 7 years (wave 4). Information was collected via face to face interviews as well as self-completed questionnaires. The study began with an original sample of 18,818 cohort members, with 14,043 cohort members remaining at wave 4. This study uses exposure and covariate data from the first wave, and outcome and covariate data from the fourth wave. Ethical approval for the MCS was granted from the London Multicentre Research Ethics Committee. All survey materials are available online (www.cls.ioe.ac.uk/mcs).
The study cohort consisted of singleton births who were recruited into the MCS cohort at 9 months of age, were present at the age 7 survey (wave 4) and answered any of the three asthma questions; ‘Ever asthma’, ‘Wheezing in the last 12 months’ or ‘Taking regular medication (for asthma)’. Children were excluded if the natural mother was not the main respondent at the 9 months (wave 1) interview.
Exposure measure
HDP was measured at the first wave of the MCS when the offspring were 9 months old using a two-tiered question. Firstly, women were asked if they had suffered from ‘any illnesses or other problems during pregnancy that required medical attention or treatment’. Those that answered ‘yes’ to this question were then asked to choose from a list of illnesses which included ‘raised blood pressure, eclampsia/pre-eclampsia or toxaemia’. Women who indicated this were considered to have a HDP. There were no questions relating to individual HDPs in the survey.
Outcome measures
At the fourth wave, when the children were 7 years old, mothers were asked a series of questions about asthma and wheezing including occurrence, frequency and severity indicators for each child. These questions were taken from a validated questionnaire, the International Study of Asthma and Allergies in Childhood (ISAAC) core questionnaire. This validated questionnaire has been widely used to measure childhood asthma and wheezing illnesses.20,21 In this study the primary outcome was parent-reported diagnosis of asthma in the offspring (ever asthma) by age seven. Secondary outcomes were parent-reported ‘wheezing in the last 12 months’ and regular use (every day for two weeks or more) of anti-asthmatic medications by the child at age seven. A child was recorded as taking asthma medication if they were prescribed one or more medications in the following categories based on British National Formulary Codes: Bronchodilators such as Salbutamol, Corticosteroids such as Fluticasone, and Leukotriene Receptor Antagonists such as Montelukast.
Potential confounding factors
A range of potential confounding factors was identified a priori; (1) Sociodemographic factors: ethnicity, maternal age at birth, family poverty status (defined as income under 60% of OECD national median) and maternal education; (2) Birth related factors: birth order and sex of the offspring; (3) Other established risk factors for asthma: maternal history of asthma,22 maternal smoking during pregnancy23 and maternal pre-pregnancy body mass index.24
Data analysis
Logistic regression models were used to examine the association between HDP and each outcome. For each outcome, four logistic regression models were performed. Firstly, we performed crude analysis (model 1). Second, we adjusted for a range of sociodemographic factors including maternal age, ethnicity, maternal education, poverty and maternal asthma, which we considered the main model (model 2). Third, we adjusted for maternal smoking in pregnancy and maternal BMI (model 3). Finally, we controlled for birth related factors including birth order and sex of the offspring (model 4). Children who had missing data on potential confounders were categorised into a separate group using the missing data indicator method.
To examine the effect of small for gestational age (SGA) (calculated using a customised centile calculator accounting for maternal height and weight, ethnicity, birth order, sex, gestational age and birthweight)25 on the association between HDP and child outcomes, we created a variable with the following four categories; (1) no HDP and no SGA (reference group); (2) HDP but no SGA; (3) HDP and SGA (concurrent); (4) SGA but no HDP. Although the main focus in this study is on HDP, we report the results of SGA only for completeness. Similarly, we created a variable for HDP and preterm delivery (born alive before 37 weeks of pregnancy completed) with the following four categories; (1) term delivery without HDP; (2) HDP and term delivery; (3) HDP and preterm delivery; and (4) preterm delivery without HDP. For completeness, we also conducted analysis restricted to those born term and not SGA in our supplementary material. The relationship between HDP, SGA and preterm delivery is complex. We considered that SGA and preterm birth may act as both mediators and confounders of the HDP-asthma association. For example, if HDP precedes SGA or preterm delivery, the latter variables may act as mediators of the HDP-asthma association. However, it is also plausible that women who experience preterm delivery may not have had sufficient time during pregnancy to develop HDP. Moreover, SGA or IUGR may underlie cases of iatrogenic preterm delivery. Some of these women may have gone on to develop HDP (e.g. pre-eclampsia or gestational hypertension) if they had not been delivered early. Thus, they may also play a role as potential confounders.
Sensitivity analysis
We sought to examine the effect of maternal history of atopy (asthma, eczema or hayfever) on the association between HDP and child outcomes. We report the results of the association between HDP and child outcomes for children with and without maternal atopy separately. All analysis was completed using SPSS, Version 24.
Results
A total of 18,552 families (18,818 children) participated in the first wave of this survey, including 18,241 singleton births whose natural mother was the main respondent at the first interview. Of these, 13,163 (72.2%) participated in the fourth wave and 13,089 (71.8%) answered at least one of the three asthma questions (‘Ever asthma’, ‘Wheezing in the last 12 months’ or ‘Taking regular medication’) and were included in the analysis (Fig 1). Sixteen per cent (n = 2151) of children had developed asthma by age seven years, 12% (n = 1585) reported wheezing in the past 12 months and 4% (n = 523) of the cohort were taking anti-asthmatic medications regularly. Eight per cent (n = 986) of mothers reported having HDP at the first wave of the MCS.
Inclusion criteria were singleton births who were present at the age 7 survey and answered any of the three asthma questions; ‘Ever asthma’, ‘Wheezing in the last 12 months’ or ‘Taking regular medication (for asthma)’. Children were excluded if the natural mother was not the main respondent at the 9 month interview.
Parent and child characteristics are presented in Table 1. Boys were more likely to have asthma by age 7 than girls (19.2% vs 13.7%). Children with parent-reported asthma by age 7 were more likely to have a family history of asthma, to be born to parents who were less wealthy or less educated, to be born to mothers who were younger, obese or smoked during pregnancy, to be born preterm, and were less likely to be breast fed than children without asthma. For completeness, baseline characteristics of those who dropped out of the MCS by wave four are included in Supplementary Table 1.
HDP and risk of asthma in the offspring
In the crude logistic regression model, HDP was significantly associated with asthma (OR 1.37; [95% CI: 1.17–1.61]) and the association did not change materially when the model was adjusted for maternal age, ethnicity, education, socioeconomic status and maternal asthma (model 2) (AOR 1.35; [95% CI: 1.15–1.59]). The association remained almost unchanged in further models adjusting for maternal BMI, maternal smoking and birth related factors (Table 2 and Supplementary Table S2). In a post-hoc analysis, we did a subgroup analysis to examine the association between HDP and the odds of ever asthma by age 7 (Supplementary Table S3). The OR was higher for males than females, however, the difference between the two ORs was not statistically significant (OR = 1.25; [95% CI: 0.96–1.62] for females; and OR = 1.42; [95% CI: 1.14–1.75] for males; p-value for interaction 0.46).
Combined analysis of HDP and SGA/preterm birth
We examined the odds of asthma in children exposed to HDP with or without concurrent SGA. Children who were exposed to HDP and born appropriate for gestational age (AGA) had higher odds of asthma (AOR 1.27; [95% CI: 1.05–1.53]) compared with AGA children not exposed to HDP. However, the odds of asthma were further increased among children exposed to concurrent HDP and SGA (AOR 1.58; [95% CI: 1.15–2.18]).
Similarly, we examined the odds of asthma in children born preterm and exposed to HDP. Children who were exposed to HDP and born at term had increased odds of asthma compared with those unexposed to HDP (AOR 1.32; [95% CI: 1.10–1.58]). However, the highest odds of asthma were observed among children born preterm and exposed to HDP (vs. term delivery without HDP, AOR 1.81; [95% CI: 1.25–2.61]) (Table 3).
We then completed an exploratory post-hoc analysis examining the odds of asthma in children exposed to HDP restricting the analysis to those born at term (AOR 1.32; [1.10–1.59]) and those born AGA (AOR 1.28; [1.06–1.54]) (Supplementary Table S4).
HDP and risk of recent wheeze or use of anti-asthmatic medication
On crude analysis, children exposed to HDP were significantly more likely to experience recent wheeze and take anti-asthmatic medications regularly; OR 1.28; [95% CI: 1.06–1.54] and OR 1.34; [95% CI: 1.00–1.81], respectively (Table 2). After adjustment for a range of confounding factors including maternal age, ethnicity, maternal education, poverty and familial asthma, the associations were slightly attenuated, but remained significant for recent wheeze (AOR 1.26 [95% CI: 1.04–1.51]). In further models adjusting for maternal BMI, maternal smoking and birth related factors the results remained largely unchanged (Supplementary Table S2). For completeness, we undertook an analysis investigating the association between HDP and a combination of; 1. Asthma plus wheeze and; 2. Asthma plus regular use of anti-asthmatic medications. This analysis did not show any meaningful difference from our main results (Supplementary Table S5).
Sensitivity analysis
When only individuals with a maternal history of atopy (asthma, eczema or hayfever) were included in our analysis the association between HDP and asthma in the offspring remained significant, as per the main cohort results (AOR 1.32; [95% CI: 1.06–1.64]) (Table 4).
Discussion
HDP and asthma
In this population-based prospective cohort study involving over 13,000 individuals, we identified a positive association between HDP and asthma among seven year old children. This association remained consistent after adjustment for several potential confounders including sociodemographic factors, birth-related factors and known risk factors for asthma. Interestingly, we found that when the offspring were born SGA or preterm, and exposed to HDP, a larger effect estimate was evident, which is consistent with recently published data.10,12
Sensitivity analysis revealed that when the analysis was restricted to individuals with a family history of atopy the association between HDP and asthma remained significant, as per the main cohort results. Familial aggregation of asthma and atopic disease has frequently been noted, with studies finding numerous candidate genes, epigenomic modifications and chromosomal regions playing a role in the development of asthma,26,27 most pronounced when there is a maternal history of asthma28,29 which was adjusted for in our main analysis. The fact that the association remained significant in this high-risk group strengthens the results of our study.
Comparison with previous studies
Results from previous studies examining the effect of HDP on the development of asthma in the offspring have been inconsistent.9,10,11,12,13,14,15,16,17 Two large Danish9 and Norwegian10 studies involving over 500,000 individuals found a positive association between pre-eclampsia and asthma in the offspring. The overall association was weakly positive, and attenuated to null after sibling-matched analysis.
In our study, sensitivity analysis revealed that the OR of the association between HDP and asthma remained significant among children with a maternal history of atopy. This is consistent with another large Danish Study,11 which found an association between pre-eclampsia and ongoing treatment with inhaled corticosteroids at age 7 years among children whose mothers had doctor-diagnosed asthma. Interestingly, in the aforementioned three studies9,10,11 the definition of asthma was not based on a validated tool but instead based on hospitalisations for asthma during a 14 year period,9 dispensed asthma medication in the past 12 months10 or clinical investigator diagnosed endpoints.11 Whilst these definitions may account for severe cases of asthma, they may exclude milder cases of asthma and may not be representative of all asthma cases in the general population. Furthermore, clinical investigator diagnosed endpoints may lead to subjective bias in the diagnosis of asthma.
More recently, a prospective study in the Netherlands13 investigated the association between individual blood pressure readings in pregnancy and HDP (gestational hypertension and pre-eclampsia) with asthma at age 10 years. They found a positive association between high maternal blood pressure measurements in late pregnancy and asthma at age 10, but no association between HDP diagnosis and asthma at age 10. It was not explicitly stated whether individuals with HDP were excluded from their analysis of blood pressure measurements in late pregnancy. Thus, it is plausible that uncontrolled hypertension in late pregnancy may increase the risk of asthma in childhood. A recent British prospective study12 involving 6974 participants investigated the relationship between HDP and asthma at 7 years. They reported a positive association between chronic hypertension, but not gestational hypertension or pre-eclampsia, and asthma in the offspring. Further research is required to elucidate the association between individual HDP and asthma risk in the offspring.
Potential mechanisms
A potential explanation for the positive association found between HDP and asthma in the offspring may be the influence of immunological mechanisms, specifically in women with pre-eclampsia. Immune tolerance in pregnancy plays an important role in healthy pregnancy outcomes. Studies are now suggesting that pre-eclampsia may be in part due to maladaptation of this maternal immune tolerance.30,31,32 One may speculate that maladaptation of this immune tolerance may have an effect on the offspring’s development of immune-related diseases such as asthma.10
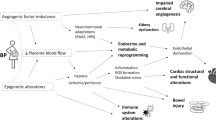
Furthermore, HDP are characterised by an imbalance in circulating angiogenic factors.33 One recent study suggests that the angiogenic factor imbalance in the mother is reflected in their offspring with neonates born to mothers with pre-eclampsia having higher levels of cord blood soluble fms-like tyrosine kinase-1 (sFlt-1) and lower levels of placental growth factor and vascular endothelial growth factor (VEGF) levels.34 Low levels of VEGF35,36 and high levels of sFlt-137 have been shown to reduce alveolarization and lead to persistent abnormalities of pulmonary vascular structures in animal studies, which in turn may potentially lead to decreased lung function in childhood.
It is well documented that HDP are associated with low birthweight and preterm delivery,38,39 which themselves are independently associated with wheezing, asthma and impaired lung function in children.40,41,42,43,44 The present findings which suggested that the interaction between SGA and HDP and preterm birth and HDP have a larger effect estimate with asthma, compared to HDP only, may support a hypothesis that the association between HDP and asthma may partly be explained by SGA and preterm birth.12
Strengths and limitations
This study has several strengths. Firstly, it utilised a large population-based cohort which, due to the sampling methods employed, is representative of all children born in the UK between 2000 and 2002. Secondly, a validated questionnaire from the International Society of Asthma and Allergies in Childhood was used for our outcome measurement. This validated instrument has been widely used to measure childhood asthma and wheezing illnesses20,21 and all questions in the questionnaire have been shown to have a specificity of over 90%.45 As well as this, our study had ample information on a large number of potential confounders.
However, it does have limitations. Firstly, HDP is self-reported by mothers 9 months following delivery. This is subject to potential recall bias, although HDP has been shown to be accurately reported post-delivery in a large population-based study previously.46 Secondly, HDP was reported overall, and thus could not be examined by a specific disease or disease severity. It was not possible to identify cases of pre-eclampsia within the dataset, nor to separate early vs. late pre-eclampsia. Previous studies suggest that the association may depend on the specific hypertensive disease, although more recent studies reported that individual readings of high blood pressure in pregnancy may also increase the risk of asthma in childhood.13 Furthermore, the MCS does not have data available on maternal asthma medical level or asthma exacerbations in pregnancy. This may have an impact on results reported in Table 3 as the use of asthma medications in pregnancy could potentially have an impact on fetal growth. Similarly, paternal data was limited in the MCS such as paternal smoking and paternal history of atopic disease. However, it is unlikely that these two variables are associated with maternal HDP and therefore unlikely to confound the association between HDP and the outcome measures. Furthermore, there was a large amount of missing data on these factors in the MCS cohort. It is important to note that the relationship between SGA and HDP is rather complex. For the purposes of our analysis, we considered that SGA may act as both a mediator and a confounder. This was because IUGR may precede iatrogenic preterm delivery which, in some cases, may prevent women from developing HDP—particularly if they deliver very/extremely preterm. Similarly, preterm birth could have a potential confounding or mediating role between HDP and child atopic disease. To avoid over adjusting the regression models, we conducted separate models to assess the role of SGA and preterm birth in the association between HDP and child atopic disease. Finally, as with all cohort studies, loss to follow up is a potential limitation. 18,818 were recruited in the first wave of this study, however by sweep four there were 14,043 (74.6%) responses. For completeness, we have provided Supplementary Table S1 comparing information between those included in the study and those lost to follow up before MCS 4. The group lost to follow up were more ethnically diverse than those who participated in wave 4. This could introduce some bias into our results. However, other baseline characteristics between the two groups were similar limiting the potential for the same.
Conclusion
This study suggests that children exposed to HDP may be at increased odds of asthma diagnosis by age seven years, particularly if they were also born SGA or preterm. Further studies examining subgroups of HDP and their effect on asthma risk in the offspring are warranted.
Data availability
The data from this study were from the Millennium Cohort Study and can be obtained by applying to the UK Data Service. Further details are available at https://beta.ukdataservice.ac.uk/datacatalogue/series/series?id=2000031
References
Patel, S., Järvelin, M. & Little, M. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ. Health 7, 57 (2008).
Carson, C. et al. Asthma in children born after infertility treatment: findings from the UK Millennium Cohort Study. Hum. Reprod. 28, 471–479 (2012).
Sennhauser, F., Braun-Fahrländer, C. & Wildhaber, J. The burden of asthma in children: a European perspective. Paediatr. Respir. Rev. 6, 2–7 (2005).
Global Strategy for Asthma Management and Prevention Mortality and Morbidity. National Heart, Lung and Blood Institute 16–18 (NIH, Bethesda, MD, 2002).
Cruz, A. & Bousquet, P. The unbearable cost of severe asthma in underprivileged populations. Allergy 64, 319–321 (2009).
Ed.ac.uk [Internet]. https://www.ed.ac.uk/files/atoms/files/asthma-care-costs-uk-at-least-ps1.1bn-each-year-study-shows-29-08-2016.pdf. Accessed 4 March 2019 (2019).
Olson-Chen, C. & Seligman, N. Hypertensive emergencies in pregnancy. Crit. Care Clin. 32, 29–41 (2016).
Stick, S., Burton, P., Gurrin, L., Sly, P. & LeSouëf, P. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet 348, 1060–1064 (1996).
Liu, X. et al. Maternal preeclampsia and childhood asthma in the offspring. Pediatr. Allergy Immunol. 26, 181–185 (2015).
Magnus, M. C. et al. Pre-eclampsia and childhood asthma. Eur. Respir. J. 48, 1622–1630 (2016).
Stokholm, J., Sevelsted, A., Anderson, U. D. & Bisgaard, H. Preeclampsia associates with asthma, allergy, and eczema in childhood. Am. J. Respir. Crit. Care Med. 195, 614–621 (2017).
Shaheen, S., Macdonald-Wallis, C., Lawlor, D. & Henderson, A. Hypertensive disorders of pregnancy, respiratory outcomes and atopy in childhood. Eur. Respir. J. 47, 156–165 (2015).
Wilmink, F. et al. Maternal blood pressure and hypertensive disorders during pregnancy and childhood respiratory morbidity. The Generation R Study. Eur. Respir. J. 52, 1800378 (2018).
Byberg, K., Ogland, B., Eide, G. & Øymar, K. Birth after preeclamptic pregnancies: association with allergic sensitization and allergic rhinoconjunctivitis in late childhood; a historically matched cohort study. BMC Pediatr. 14, 101 (2014).
Nafstad, P. et al. Pregnancy complications and the risk of asthma among Norwegians born between 1967 and 1993. Eur. J. Epidemiol. 18, 755–761 (2003).
Zugna, D. et al. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int. J. Epidemiol. 44, 199–208 (2015).
Annesi-Maesano, I., Moreau, D. & Strachan, D. In utero and perinatal complications preceding asthma. Allergy 56, 491–497 (2001).
Plewis, I. The Millennium Cohort Study: Technical Report on Sampling (Centre for Longitudinal Studies, London, 2007.
Connelly, R. & Platt, L. Cohort profile: UK Millennium Cohort Study (MCS). Int. J. Epidemiol. 43, 1719–1725 (2014).
Asher, M. et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur. Respir. J. 8, 483–491 (1995).
Asher, M. I. & Stewart, A. W., On Behalf of the International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur. Respir. J. 12, 315–335 (1998).
Burke, W., Fesinmeyer, M., Reed, K., Hampson, L. & Carlsten, C. Family history as a predictor of asthma risk. Am. J. Prev. Med. 24, 160–169 (2003).
Thacher, J. D. et al. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics 134, 428–434 (2014).
Reichman, N. & Nepomnyaschy, L. Maternal pre-pregnancy obesity and diagnosis of asthma in offspring at age 3 years. Matern. Child Health J. 12, 725–733 (2007).
Gardosi, J. & Francis, A. in v6.7 (UK). Gestation Network (ed. GROW) (2013).
Edris, A., den Dekker, H., Melén, E. & Lahousse, L. Epigenome‐wide association studies in asthma: a systematic review. Clin. Exp. Allergy 49, 953–968 (2019).
Tizaoui, K., Hamzaoui, K. & Hamzaoui, A. Update on asthma genetics: results from meta-analyses of candidate gene association studies. Curr. Mol. Med. 17, 647–667 (2018).
Lim, R., Kobzik, L. & Dahl, M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS ONE 5, e10134 (2010).
Medsker, B. et al. Maternal depressive symptoms, maternal asthma, and asthma in school-aged children. Ann. Allergy Asthma Immunol. 118, 55–60.e1 (2017).
Hsu, P. & Nanan, R. K. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front. Immunol. 5, 125 (2014).
Redman, C. W. & Sargent, I. L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 63, 534–543 (2010).
Wilczynski, J. R. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia – the same basic mechanism? Hum. Immunol. 67, 492–511 (2006).
Leaños-Miranda, A. et al. Circulating angiogenic factors are related to the severity of gestational hypertension and preeclampsia, and their adverse outcomes. Medicine 96, e6005 (2017).
Tsao, P. N. et al. Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics 116, 468–472 (2005).
Le Cras, T. D., Markham, N. E., Tuder, R. M., Voelkel, N. F. & Abman, S. H. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L555–L562 (2002).
Jakkula, M. et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L600–L607 (2000).
Tang, J. R., Karumanchi, S. A., Seedorf, G., Markham, N. & Abman, S. H. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L36–L46 (2012).
Xiong, X. et al. Impact of pregnancy-induced hypertension on fetal growth. Am. J. Obstet. Gynecol. 180, 207–213 (1999).
Geelhoed, J. et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures. Circulation 122, 1192–1199 (2010).
Rona, R. J., Gulliford, M. C. & Chinn, S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ 306, 817–820 (1993).
Jaakkola, J. J. K. et al. Preterm delivery and asthma: a systematic review and meta-analysis. J. Allergy Clin. Immunol. 118, 823–830 (2006).
Kotecha, S. J. et al. Spirometric lung function in school-age children: effect of intrauterine growth retardation and catch-up growth. Am. J. Respir. Crit. Care Med. 181, 969–974 (2010).
Kotecha, S. J. et al. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax 67, 54–61 (2012).
Sonnenschein-van der Voort, A. et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J. Allergy Clin. Immunol. 133, 1317–1329 (2014).
Lukrafka, J. et al. Performance of the ISAAC questionnaire to establish the prevalence of asthma in adolescents: a population-based study. J. Asthma 47, 166–169 (2010).
Falkegård, M., Schirmer, H., Løchen, M., Øian, P. & Acharya, G. The validity of self-reported information about hypertensive disorders of pregnancy in a population-based survey: the Tromsø Study. Acta Obstetr. Gynecol. Scand. 94, 28–34 (2014).
Acknowledgements
We would like to thank all the children and their families who participate in the MCS.
Funding
This work was supported by the Academic Internship Track Bursary, University College Cork, Cork, Ireland & Health Service Executive. LK was an Academic Intern in receipt of this funding.
Author information
Authors and Affiliations
Contributions
ASK, PB and LK planned the study. LK carried out the analysis and wrote the manuscript. PB, FM, GWOK, GM and ASK took part in drafting the article or revising it for critically important intellectual content and all authors gave final approval of this version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Patient consent was not required for this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kelly, L., Barrett, P., McCarthy, F.P. et al. The association between hypertensive disorders of pregnancy and childhood asthma. Pediatr Res 92, 1188–1194 (2022). https://doi.org/10.1038/s41390-022-01935-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01935-x