Abstract
Background
Inflammatory and endothelial activation responses during extracorporeal membrane oxygenation (ECMO) support in children are poorly understood. In this study, we aimed to determine if circulating inflammatory, endothelial activation, and fibrinolytic markers are associated with mortality and with neurologic outcomes in children on ECMO.
Methods
We conducted a secondary analysis of a two-center prospective observational study of 99 neonatal and pediatric ECMO patients. Inflammatory (interferon gamma [IFNγ], interleukin-6 [IL-6], IL-1β, tumor necrosis factor alpha [TNFα]), endothelial activation (E-selectin, P-selectin, intercellular adhesion molecule-3 [ICAM-3], thrombomodulin [TM]), and fibrinolytic markers (tissue plasminogen activator [tPA], plasminogen activator inhibitor-1 [PAI-1]) were measured in plasma on days 1, 2, 3, 5, 7, and every third day thereafter during the ECMO course.
Results
All ECMO day 1 inflammatory biomarkers were significantly elevated in children with abnormal vs. normal neuroimaging. ECMO day 1 and peak levels of IL-6 and PAI-1 were significantly elevated in children who died compared to those who survived to hospital discharge. Tested biomarkers showed no significant association with long-term neurobehavioral outcomes measured using the Vineland Adaptive Behavioral Scales, Second Edition.
Conclusions
High levels of circulating inflammatory, endothelial activation, and fibrinolytic markers are associated with mortality and abnormal neuroimaging in children on ECMO.
Impact
-
The inflammatory, endothelial activation, and fibrinolytic profile of children on ECMO differs by primary indication for extracorporeal support.
-
Proinflammatory biomarkers on ECMO day 1 are associated with abnormal neurologic imaging in children on ECMO in univariable but not multivariable models.
-
In multivariable models, a pronounced proinflammatory and prothrombotic biomarker profile on ECMO day 1 and longitudinally was significantly associated with mortality.
-
Further studies are needed to identify inflammatory, endothelial, and fibrinolytic profiles associated with increased risk for neurologic injury and mortality through potential mediation of bleeding and thrombosis.
Similar content being viewed by others
Introduction
Extracorporeal membrane oxygenation (ECMO), initially employed in the 1970s, is a well-established form of mechanical support for severe cardiopulmonary failure. While ECMO is used increasingly in intensive care units in the U.S. and internationally,1,2 survival has remained relatively unchanged at around 55% of all ECMO cases,1,3 and neurologic injury remains a significant challenge impacting both survival and long-term outcomes.4,5,6
Neurologic injury manifested as hypoxic-ischemic injury, intracranial hemorrhage, thromboembolic stroke, or brain death has been reported in as many as 15−36% of ECMO patients.7,8,9,10,11,12 The pathophysiology of neurologic injury in patients on ECMO is multifactorial. Pre-ECMO profound cardiopulmonary failure with its corollary of hypoxia, hypotension, and acidosis can lead to hypoxic-ischemic injury, rendering the brain vulnerable.11,13,14,15 The profound inflammatory state associated with critical illness is then compounded by exposure to the foreign surfaces of the extracorporeal circuit, which triggers a global innate immune response by activating the contact and complement systems,12,13,16 and provokes perturbations in proinflammatory and prothrombotic pathways implicated in the pathogenesis of ECMO-associated neurologic injury.10,12,17 The device-induced proinflammatory state and endothelial cell dysfunction increase thrombin generation and platelet activation.18,19 Within the patient, the device contributes to coagulation factor consumption, platelet exhaustion, and reduced platelet aggregation potential.18,19 Added to that is the need for systemic anticoagulation to prevent circuit thrombus formation, creating the common paradox of thrombotic events in the device with simultaneous bleeding in the patient.20 Thus, patients are at risk for hypoxic brain injury as well as thromboembolic and hemorrhagic intracerebral complications.
In this secondary analysis of a two-center prospective observational cohort of critically ill children requiring ECMO support,7 we examined circulating inflammatory, endothelial activation, and fibrinolytic markers at ECMO initiation and serially during the ECMO course. We hypothesized that children on ECMO who develop neurological complications or have worse outcomes (in-hospital mortality or unfavorable neurobehavioral outcomes among survivors) would have increased concentrations of proinflammatory, endothelial activation, and fibrinolytic markers in plasma compared to those without neurological complications and with survivors with favorable neurobehavioral outcomes up to 1 year post ECMO.
Materials and methods
Study design and patient selection
We conducted a retrospective analysis of an observational cohort study. Patients were prospectively enrolled within 24 h of ECMO initiation at two academic, urban, pediatric intensive care units. Inclusion and exclusion criteria as well as enrollment details have been described previously.7 All patients enrolled in the parent cohort were eligible for this study. This study was approved by the Institutional Review Boards at both participating sites.
Demographic, clinical, and neuroimaging data were collected prospectively during the hospitalizations that included the ECMO course. The primary outcome was neurological complications (diffuse hypoxic injury, intracranial hemorrhage, and ischemic stroke) determined by neuroimaging. Neuroimaging studies obtained by the clinical team as part of institutional clinical protocols or for clinical indications, included head ultrasound (obtained pre-ECMO and daily during the ECMO course at both sites), brain computed tomography (CT), and brain magnetic resonance imaging (MRI). The date, time, and type of new neuroimaging abnormality were recorded for all neuroimaging studies available during ECMO and up to 6 weeks after decannulation. Secondary outcomes included in-hospital mortality, and, among survivors to hospital discharge, the Vineland Adaptive Behavior Scales-II (VABS-II) score dichotomized at 85 (i.e., −1 SD below the reference population mean).
ECMO circuit components
The ECMO circuits during the study period consisted of custom-packed 1/4- or 3/8-inch flexible PVC tubing (Medtronic, Minneapolis, MN) with a silicone reservoir, a bladderbox (Johns Hopkins Hospital, Baltimore, MD), a 0.8–4.5 m2 membrane oxygenator (Medtronic), a heat exchanger (Medtronic), and a roller pump (Sorin Cardiovascular, Arvada, CO) up to January 2011, and the Better Bladder (Coastal Life Systems Inc, San Antonio, TX), the Quadrox-ID oxygenator (Maquet Cardiopulmonary, Rastatt, Germany), and a roller or centrifugal pump (Sorin), thereafter, at one site. At the other site, the ECMO circuit consisted of custom-packed 1/4- or 3/8-inch flexible PVC tubing (Medtronic, Minneapolis, MN), the Better Bladder (Circulatory Technology, Inc), the Quadrox-ID oxygenator (Getinge Cardiopulmonary, Rastatt, Germany), and a centrifugal pump (Getinge Cardiopulmonary, Rastatt, Germany). Catheters used at both institutions include Bio-Medicus One-Piece Femoral Arterial Cannula (Medtronic), Bio-Medicus One-Piece Femoral Venous Cannula (Medtronic), OriGen reinforced dual lumen catheter (OriGen), and Avalon Elite Bi-Caval Dual Lumen Catheter (Getinge). One site also used Percutaneous Sheath Introducer Kit (reperfusion cannula) (Arrow), while the other institution also used DLP Pediatric One-Piece Arterial Cannula (Medtronic) and DLP Malleable Single Stage Venous Cannula (Medtronic).
Long-term outcome assessment
VABS-II assessment was conducted in person by trained study personnel at each site at 6 months and 1 year post ECMO. The VABS-II instrument measures adaptive behavior skills and provides age-corrected standard scores from birth to 18 years of age for overall adaptive behavior composite (mean = 100, SD = 15) and four domains (motor skills, socialization, daily living, and communication). Higher scores are reflective of higher adaptive behavior function. Study participants who survived to discharge were stratified by status at 6-month and 1-year follow-up and those who did not complete a follow-up visit.
Biomarker sampling and analysis
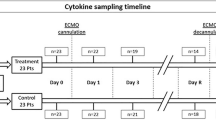
Blood samples were obtained daily during ECMO from the ECMO circuit or existing indwelling catheters, at the same time as routine clinical blood draws. Plasma was separated by centrifugation and stored at −80 °C. Plasma concentrations for interferon gamma (IFNγ), interleukin-6 (IL-6), interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNFα), E-selectin, P-selectin, intercellular adhesion molecule-3 (ICAM-3), thrombomodulin (TM), tissue plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1) were measured for ECMO days 1, 2, 3, and 5. Measurements for ECMO day 7 and every third day thereafter until ECMO decannulation was conducted if ECMO duration was longer than 5 days.
All assays were conducted in the Johns Hopkins Institute for Clinical and Translational Research (ICTR) Clinical Research Unit: Core Laboratory, using the Meso Scale Discovery (Rockville, MD) V-PLEX Human Proinflammatory Panel (for IFNγ, IL-6, IL-1β, TNFα), the Human Vascular Injury I Kit (for E-selectin, P-selectin, ICAM-3, TM), and single plex assays for tPA and PAI-1. Dynamic ranges for each analyte have been published.21,22,23 The inter-assay coefficients of variation for the two multiplex assays used were: IFNγ, 7.96; IL-6, 15.93; IL-1β, 0.02; TNFα, 5.21; E-selectin, 6.45; P-selectin, 14.39; ICAM-3, 11.78; TM, 8.6. Laboratory personnel were blinded to clinical data.
Statistical analysis
For each biomarker, we characterized the distributions of the first measured sample (within 24 h of ECMO cannulation). The ECMO day 1 biomarker level may be most clinically relevant as an initial level collected prospectively prior to specific ECMO complications and outcomes. Distributions were stratified by ECMO indication, neurological complications, in-hospital mortality status, and VABS-II score ≥85 up to 1 year among those who survived to discharge. Distributions were graphically depicted by percentile boxplots and were compared using the Wilcoxon rank sum test.
To estimate the association of differences in ECMO day 1 and longitudinal biomarker concentrations and the risk of in-hospital mortality, separate Cox proportional hazards models were fit for each biomarker to provide time-constant hazard ratios (HRs). Those who survived to hospital discharge were treated as censored, and the HRs are therefore interpreted as cause-specific HRs. This modeling strategy circumvented tethering inherent in estimating subdistribution HRs and was better supported by this smaller dataset.24,25 In this model, the independent variables were the biomarker on ECMO day 1, and the difference of the biomarker from this first day at each subsequent longitudinal measurement. All biomarkers were log2 transformed. The HRs are interpreted as the between-individual risk of in-hospital mortality associated with a twofold higher level from ECMO day 1 (adjusting for longitudinal changes), and the risk associated with a subsequent doubling of the biomarker from ECMO day 1 (adjusting for ECMO day 1 level). For consistency across biomarkers, we estimated risk associated with a twofold between- and within-person difference, which was very close to a standard deviation of the biomarker distributions. The models were additionally adjusted for age (neonate vs. non-neonate) and primary ECMO indication (respiratory vs. non-respiratory).
To measure the risk of abnormal neuroimaging during or following the ECMO course, we used Cox proportional hazards models similar to those described above, with time to abnormal neuroimaging as the outcome. These models describe the risk associated with higher ECMO day 1 and longitudinal biomarker levels, adjusted for age and primary ECMO indication. Those who did not have neuroimaging (n = 11) or whose imaging was abnormal prior to biomarker sample collection (n = 10) were excluded from this part of the analysis.
As a supplementary analysis, we characterized the distributions of peak levels, which could have occurred at any time point during ECMO, and are assumed to reflect how biomarkers were maximally modified by disease course while on ECMO. These distributions were stratified by the same variables described above.
Statistical significance was assessed at p < 0.05. All analyses were conducted in R 3.6.0 (R Core Team, Vienna, Austria).
Results
Full demographic and clinical characteristics have been described previously7 and are also summarized in Table 1. Briefly, we enrolled 99 patients at two centers. Fifty-one (52%) study participants were neonates (<30 days), one third (33%) underwent ECMO support for primary respiratory failure, and 66 (67%) underwent ECMO for non-respiratory primary indications, including cardiac failure (39%), extracorporeal cardiopulmonary resuscitation (ECPR) (19%), and sepsis (8%). Eighty-eight (89%) children had neuroimaging during ECMO or within 6 weeks from ECMO decannulation. Forty-four of 88 (50%) children with neuroimaging showed evidence of embolic infarction, intracranial hemorrhage, or postasphyxial brain injury. Forty-two (42%) children died prior to hospital discharge. Plasma collected for research purposes was available for this study in 97/99 (98%) of study participants. Plasma biomarker concentrations on ECMO day 1 and the highest concentration observed during the ECMO course (peak levels) are presented in Supplementary Table 1.
ECMO day 1 and peak biomarkers by ECMO indication
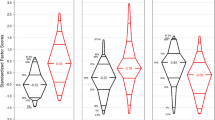
Day 1 biomarker levels in children on ECMO for primary respiratory failure were compared to children on ECMO for non-respiratory indications (Fig. 1). IL-6 (median 65.0 vs. 17.5 pg/mL), thrombomodulin (median 5.4 vs. 4.4 ng/mL), tPA (median 7.4 vs. 4.2 ng/mL), and PAI-1 (median 200.3 vs. 30.0 ng/mL) were significantly elevated in children on ECMO for non-respiratory indications compared to those with respiratory failure. Median peak biomarker levels all increased from day 1 regardless of diagnosis, with the exception of P-selectin for which peak and day 1 were similar. Peak levels were significantly elevated in children supported on ECMO for non-respiratory indications compared to those on ECMO for respiratory failure for IL-6 (median 135.6 vs. 19.1 pg/mL), TNFα (median 6.6 vs 4.9 pg/mL), thrombomodulin (median 7.6 vs 6.5 ng/mL), tPA (median 10.2 vs 6.0 ng/mL), and PAI-1 (median 284.3 vs. 54.6 ng/mL) (Fig. 1).
ECMO day 1 biomarker level for primary respiratory indication (black solid circles) vs. non-respiratory indication (gray solid circles), peak biomarker level for primary respiratory indication (black solid triangles) vs. non-respiratory indication (gray solid triangles). Biomarkers represent underlying inflammatory, endothelial activation, and fibrinolytic processes. Wilcoxon rank sum p values (* indicates p < 0.05, ** indicates p < 0.001) are presented contrasting differences in location between respiratory failure and non-respiratory indications for ECMO.
ECMO day 1 and peak biomarkers by neurological complications
Forty-three of 44 patients who had abnormal findings on neuroimaging had biomarker concentrations evaluated. Evaluation of biomarkers in children with abnormal vs. normal neuroimaging revealed ECMO day 1 levels to be significantly elevated for all inflammatory biomarkers (IFNγ, median 17.5 vs. 9.0 pg/mL; IL-6, median 57.9 vs. 26.5 pg/mL; IL-1β; 0.4 vs. 0.3 pg/mL; TNFα, 4.7 vs. 3.2 pg/mL) (Fig. 2). Peak levels remained significantly elevated only for TNFα (median 6.5 vs. 5.1 pg/mL) in children with abnormal neuroimaging compared to normal neuroimaging (Fig. 2).
ECMO day 1 biomarker level with normal neuroimaging (black solid circles) vs. abnormal neuroimaging (gray solid circles), peak biomarker level with normal neuroimaging (black solid triangles) vs. abnormal neuroimaging (gray solid triangles). Wilcoxon rank sum p values (* indicates p < 0.05) are presented contrasting differences in location between those with abnormal vs. normal neuroimaging.
ECMO day 1 and peak biomarkers by mortality and long-term outcomes
Of the 42 children who died prior to hospital discharge, 41 had plasma samples available. ECMO day 1 concentrations were significantly elevated in children who died vs. those who survived for IL-6 (median 98.5 vs. 23.1 pg/mL) and PAI-1 (median 200.3 vs. 94.6 ng/mL) and significantly decreased for E-selectin (13.9 vs. 18.0 ng/mL) (Fig. 3). Peak biomarker concentrations remained significantly elevated for IL-6 (median 102.8 vs. 32.6 pg/mL) and PAI-1 (330.3 vs. 99.5 ng/mL) in those who died compared to those who survived. Peak concentrations of tPA (median 9.8 vs. 8.3 ng/ml) were also significantly higher in children who died compared to those who survived (Fig. 3).
ECMO day 1 biomarker level in those who survived (black solid circles) vs. those who died (gray solid circles), peak biomarker level in those who survived (black solid triangles) vs. those who died (gray solid triangles). Wilcoxon rank sum p values (* indicates p < 0.05, ** indicates p < 0.001) are presented contrasting differences in location between those who survived and those who died in hospital.
Among survivors to hospital discharge, day 1 and peak biomarker concentrations during the ECMO course were similar for children who at 6- and/or 12-month follow-up had a VABS-II composite score of ≥85, <85, or post-discharge mortality, or no follow-up, respectively.
Multivariable models of ECMO day 1 and longitudinal biomarker trajectory and outcomes
To explore the relationship between biomarker levels on ECMO day 1 and longitudinally, cause-specific hazard ratios were estimated from Cox proportional hazards models, with adjustment for age and primary indication for ECMO.
In these multivariate models, between-person twofold higher biomarker levels on ECMO day 1 and within-person doubling of biomarker levels over time, respectively, were not significantly associated with abnormal neuroimaging during or following the ECMO course.
In multivariable Cox proportional hazards models, higher inflammatory markers on ECMO day 1 were associated with in-hospital mortality, with the relationships for IL-6, IL-1β, and TNFα being statistically significant (Fig. 4). Between-person twofold higher ECMO day 1 IL-6, IL-1β, and TNFα concentrations were associated with higher hazard for mortality: adjusted HR (aHR), 1.22 (95% CI: 1.07, 1.39), 1.38 (95% CI: 1.13, 1.70), and 1.45 (95% CI: 1.07%, 1.96), respectively. Within-person twofold increase from day 1 of IL-1β and TNFα concentrations during the ECMO course was associated with a higher hazard for mortality: aHR, 1.24 (95% CI: 1.05, 1.46) and 1.36 (95% CI: 1.02, 1.83), respectively.
Risk of in-hospital mortality (a) and abnormal neuroimaging (b) associated with a twofold higher biomarker level on ECMO day 1 between patients (solid circles) and a within-patient twofold increase longitudinally (solid triangles), both expressed as adjusted hazard ratios from a multivariate Cox proportional hazards model. Models were adjusted for ECMO day 1 biomarker level, age category (neonate, infant, child, and adolescent), and ECMO indication (primary respiratory vs. non-respiratory indications).
Markers of endothelial activation were less consistent. For E-selectin, higher ECMO day 1 and longitudinal levels were associated in protective and harmful directions, respectively, and neither was significant. While higher day 1 P-selectin concentration was not strongly associated with mortality, higher longitudinal changes from day 1 were significantly associated with increased risk, although the confidence interval was wide (aHR: 1.82, 95% CI: 1.02, 3.24). ECMO day 1 and longitudinal increases of TM and ICAM-3 were near null.
Between-person twofold higher ECMO day 1 tPA was not associated with increased hazard for mortality, but a within-person twofold increase of tPA from day 1 was significantly associated with a higher hazard for mortality, aHR 1.69 (95% CI: 1.02, 2.82). For PAI-1, twofold higher day 1 levels between patients, and a within-person twofold increase from day 1, were strongly associated with in-hospital mortality, aHR 1.62 (95% CI: 1.26, 2.10), and aHR 1.73 (95% CI: 1.36, 2.21), respectively (Fig. 4).
PAI-1/tPA molar ratios
ECMO day 1 and peak PAI-1/tPA molar ratios were calculated and were significantly elevated in children on ECMO for non-respiratory vs. respiratory indications (ECMO day 1 and peak p < 0.001) as well as in those who died compared to those who survived to hospital discharge (ECMO day 1 p = 0.005; peak p < 0.001) (Supplementary Fig. 1). Twofold higher ECMO day 1 PAI-1/tPA was associated with increased hazard for mortality, aHR: 1.71 (95% CI: 1.33, 2.20) and a twofold within-person increase from ECMO day 1 was also significantly associated with mortality (aHR: 1.60, 95% CI: 1.28, 2.00).
Discussion
ECMO is regularly used as a longer-term mode of cardiopulmonary support in heterogeneous populations including patients with cardiac disease, respiratory disease, and sepsis with a high risk of neurologic injury and death.2,4,5,6,7,8,9 Patients supported on ECMO often display profound inflammation, endothelial activation, and coagulation abnormalities due to their underlying disease.16,26,27 In addition, ECMO initiation triggers further inflammation and coagulation system activation.12,13,14,15,16,28 Inflammation, endothelial activation, and coagulation abnormalities are intertwining and complex and their roles in mortality, neurologic injury, and neurologic outcomes for children on ECMO are not well understood.10,12,18,19,29,30
In this study of critically ill children on ECMO support, nonsurvivors and those with new abnormal neuroimaging findings displayed biomarker profiles indicative of more profound proinflammatory and fibrinolytic pathway activation compared to survivors and those with normal neuroimaging, respectively. After adjusting for age and primary ECMO indication, higher ECMO day 1 levels between patients and/or longitudinal increases of proinflammatory cytokines IL-6, IL-1β, and TNFα, and of fibrinolytic markers tPA and PAI-1, were associated with increased hazard for mortality but not with abnormal neuroimaging during or following the ECMO course. There were no significant biomarker differences in survivors with VABS-II scores above vs. below 85 (1 SD below the population mean) up to 1 year post-ECMO.
Significant changes in inflammatory, endothelial and fibrinolytic activation biomarkers have been described as occurring within minutes to hours of ECMO initiation regardless of indication.12,13,14,15,16 In this cohort, pre-ECMO plasma samples were not available. ECMO day 1 and peak levels of inflammatory (IL-1β, IL-6, TNFα), endothelial (TM) and fibrinolytic activation (tPA and PAI-1) biomarkers were higher in patients with a non-respiratory vs. respiratory indication for ECMO. These findings differ from those of a prior study that evaluated patterns of rise and fall of IL-1β, IL-6, IL-8, and IL-10 during ECMO, and that found no difference in these patterns among patients on ECMO with cardiac vs. respiratory etiology.27
Similar to other studies that have demonstrated increases in proinflammatory cytokines IL-1β, IL-6, and TNFα during ECMO,15,27,30,31 we observed an increase in levels during the ECMO course for all the markers evaluated, compared to day 1. When evaluating longitudinal changes in biomarkers during the ECMO course, though, it is difficult to determine if and how exposure to the ECMO circuit modulates the inflammatory and fibrinolytic responses above and beyond what would have been observed in the context of the underlying critical illness, should the patient not be on ECMO support.
Inflammatory responses in ECMO patients reflect pathophysiologic processes preceding ECMO, as well as some promoted by the ECMO circuit or care. Cytokines are often elevated in critical illnesses for which ECMO is used, including congenital diaphragmatic hernia, sepsis,32,33,34 and trauma,35 regardless of ECMO status. Different types of oxygenators36 and PaO2 levels attained during ECMO support37 also promote inflammatory responses. Proinflammatory cytokines in turn are implicated in the development of multiple organ dysfunction and are associated with worse outcomes when the cytokine cascade is amplified, leading to a hyperinflammatory phenotype.38
IL-6 induces the production of acute-phase reactants including C-reactive protein and fibrinogen, decreases albumin production, and promotes platelet activation, differentiation of CD4 and CD8 T cells, and differentiation of active B cells.39,40 Although elevations in plasma IL-6 concentrations are associated with mortality in several critically ill populations,20,35,41 data from ECMO studies are minimal. In a study conducted in 22 patients of all ages on ECMO support, Risnes et al.27 reported no significant overall differences in plasma IL-6 concentrations between survivors and nonsurvivors, although those who survived had an early peak followed by a rapid decrease in IL-6 levels within 2 days from cannulation, while those who died had persistently elevated IL-6 levels during the ECMO course.
Similar to IL-6, TNFα is a proinflammatory cytokine with many effects linking the innate and adaptive immune system. TNFα activates and promotes differentiation of macrophages, induces cytokine and prostaglandin production, increases leukocyte and endothelial activation, promotes neutrophil activation and promotes thrombosis.12,42,43 In a pediatric ECMO series (n = 16) TNFα was noted to increase significantly during ECMO in nonsurvivors compared to survivors,30 a finding not seen in our cohort.
In a study aiming to evaluate whether extracorporeal life support results in immune dysregulation, Beshish et al.44 reported serially measured plasma cytokine levels from 19 patients of all ages stratified by the presence vs. absence of immunoparalysis prior to ECMO cannulation. Plasma TNFα levels were significantly elevated in subjects with vs. those without immunoparalysis and did not change significantly over time during the ECMO course in either group. Plasma IL-6 levels showed no significant difference between subjects with vs. those without immunoparalysis. During the ECMO course, IL-6 levels remained high over time within subjects with immunoparalysis, but decreased significantly from pre-ECMO to day 3 within subjects without immunoparalysis.44 No mortality outcomes were reported in this study.44 A study by Risnes et al.27 that did not examine immune function during ECMO reported a significant decrease in IL-6 levels by day 2 in patients who survived ECMO compared to those who died. We did not evaluate the immune function status in our cohort, but similarly found that higher ECMO day 1 and higher peak plasma IL-6 levels during the ECMO course were associated with mortality. Whether persistent plasma IL-6 elevation during the ECMO course is associated with mortality or immunoparalysis remains to be determined.
Cytokines, including IL-6, IL1β, and TNFα, also play a role in coagulation activation by increasing the expression of leukocyte-adhesion molecules, activating platelets, inducing tissue factor, and increasing PAI-1 and thrombosis.18,40,45,46 In turn, cytokines can be suppressed by fibrinolytic factors such as activated protein C.47 Bleeding and thrombotic events have been associated with increased mortality among patients on ECMO.17 PAI-1 is a significant inhibitor of tPA and fibrinolysis.32,48 Together, PAI-1 and tPA form a complex that is mutually inhibitory.48 In this study, higher ECMO day 1 PAI-1 and longitudinal increase in both tPA and PAI-1 levels during the ECMO course were associated with mortality after adjusting for age and ECMO indication, although, due to the nature of the data collected in the primary study, we were not able to evaluate whether this association was mediated by bleeding and thrombotic events. PAI-1/tPA molar ratios were calculated and noted to be significantly associated with increased hazard for mortality in multivariable models. There was no significant difference in PAI-1/tPA molar ratios between those with abnormal vs. normal neuroimaging. In this study, we did not investigate the association of a shift in balance between PAI-1 and tPA and specific types of neurologic injuries that could be evaluated via brain MRI (e.g., white matter injury)49,50; however, we will plan to do so in the future studies.
IL-6, IL-1β, IFNγ, and TNFα, all proinflammatory cytokines and all noted to be elevated in patients with abnormal neuroimaging in this cohort, have been implicated in the development of brain injury.19,29,51,52 Studies have demonstrated relationships between elevated proinflammatory cytokines in cerebrospinal fluid (CSF) and serum with neurologic injury. IL-6 was shown to be elevated in the CSF of neonates with hypoxic-ischemic injury,20 in umbilical cord blood of neonates with periventricular leukomalacia,53 and in CSF and serum in adults after a stroke.51,54,55 IL-1β has also been noted to be significantly increased in CSF and serum of adults after stroke.54,55 In addition, the concentration of IL-6 in CSF was associated with the degree of neurologic injury.20,51,54 TNFα has been shown to be elevated in the amniotic fluid of neonates with periventricular leukomalacia56 but not in the CSF of neonates with hypoxic-ischemic injury.20 Despite not being significantly elevated in the CSF of subjects with stroke compared to controls, TNFα has been shown to be elevated in the CSF of adults with white matter lesions 3 months after stroke.51,55
Elevated cytokine levels have not only been associated with neurologic injury on neuroimaging but have been associated with clinical findings as well. In adults with stroke, elevations in IL-6 and TNFα were significantly elevated in those who experience clinical deterioration, described as a worsening Canadian Stroke Scale during the first 48 h post stroke.51 In term neonates who had hypoxic-ischemic injury, IL-6 was significantly increased in patients with an abnormal outcome including those who had seizures, abnormalities in tone or reflexes, or blindness.20 Neonates with white matter lesions were noted to have increased levels of TNFα, IL-1b, and IL-6 in amniotic fluid and these were correlated with the development of cerebral palsy in those who survived.56 To our knowledge no other study has evaluated the association of inflammatory, endothelial or coagulation biomarkers with neurologic outcomes in critically ill children on ECMO. Though we saw no association between these biomarkers and long-term neurobehavioral outcomes, further studies are warranted.
There were several limitations to this study. First, this was a relatively small cohort (n = 99) but it was well-characterized by several repeated samples obtained during the ECMO course. In this cohort, it was not feasible, nor was the intent to derive or validate any prediction models for risk stratification. This work is investigational in nature and thus each biomarker was evaluated individually with no adjustment done for multiple comparisons. Future models in an ongoing multicenter prospective observational cohort of neonatal and pediatric ECMO patients will be informed by the key biomarkers identified here. Secondly, we were not able to characterize disease severity and trajectory prior to ECMO initiation. The indications for ECMO were heterogeneous. Biomarker levels associated with unmeasured disease severity prior to ECMO that cause in-hospital mortality may bias the results. However, we note that biomarkers measured at the time of ECMO initiation and over the treatment course may be most clinically relevant.
In conclusion, the inflammatory and fibrinolytic profile of children on the first day of ECMO support differs by primary ECMO indication, with significantly higher IL-6, TM, tPA and PAI-1 plasma concentrations seen in children with primary cardiac, ECPR, and sepsis indications for ECMO compared to those with primary respiratory indications for ECMO. After adjusting for age, ECMO indication, and ECMO day 1 plasma concentrations for each biomarker, a within-subject doubling of IL-1β, TNFα, P-Selectin, tPA, and PAI-1 during the ECMO course was significantly associated with an increased hazard of in-hospital mortality, while only ECMO day 1 elevations of IL-6 (regardless of subsequent longitudinal trajectory) were associated with mortality. Children who eventually developed neurologic injury confirmed by neuroimaging displayed significantly elevated ECMO day 1 plasma levels of proinflammatory cytokines IL-6, IL-1β, TNFα, and IFNγ, compared to children with normal neuroimaging throughout the ECMO course and up to 6 weeks post-decannulation. However, this association became non-significant after adjusting for age and ECMO indication. Lastly, while there are limitations related to the smaller sample size of survivors to 6-month and 1-year follow-up, none of the tested biomarkers was associated with long-term neurobehavioral outcomes. Further studies are needed to investigate the association of markers of inflammation, endothelial activation, and fibrinolysis with mortality through potential mediation by bleeding and thrombosis.
References
Thiagarajan, R. R. et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 63, 60–67 (2017).
Barbaro, R. P. et al. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 63, 456–463 (2017).
Extracorporeal Life Support Organization. ELSO Registry International Summary. https://www.elso.org/Registry/Statistics/InternationalSummary.aspx (2020).
Barrett, C. S. et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr. Crit. Care Med. 10, 445–451 (2009).
Glass, P., Miller, M. & Short, B. Morbidity for survivors of extracorporeal membrane oxygenation: neurodevelopmental outcome at 1 year of age. Pediatrics 83, 72–78 (1989).
Adolph, V. et al. Developmental outcome of neonates treated with extracorporeal membrane oxygenation. J. Pediatr. Surg. 25, 43–46 (1990).
Bembea, M. M. et al. Neurologic outcomes in a two-center cohort of neonatal and pediatric patients supported on extracorporeal membrane oxygenation. ASAIO J. https://doi.org/10.1097/mat.0000000000000933 (2019).
Cengiz, P., Seidel, K., Rycus, P. T., Brogan, T. V. & Roberts, J. S. Central nervous system complications during pediatric extracorporeal life support: incidence and risk factors. Crit. Care Med. 33, 2817–2824 (2005).
Xie, A., Lo, P., Yan, T. D. & Forrest, P. Neurologic complications of extracorporeal membrane oxygenation: a review. J. Cardiothorac. Vasc. Anesth. 31, 1836–1846 (2017).
Chen, Q. et al. The effect of venovenous extra-corporeal membrane oxygenation (ECMO) therapy on immune inflammatory response of cerebral tissues in porcine model. J. Cardiothorac. Surg. 8, 1–8 (2013).
Short, B. Lou The effect of extracorporeal life support on the brain: a focus on ECMO. Semin. Perinatol. 29, 45–50 (2005).
Millar, J. E., Fanning, J. P., McDonald, C. I., McAuley, D. F. & Fraser, J. F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit. Care 20, 387 (2016).
Vallhonrat, H. et al. Rapid activation of the alternative pathway of complement by extracorporeal membrane oxygenation. ASAIO J. 45, 113–114 (1999).
Peek, G. J. & Firmin, R. K. The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J. 45, 250–263 (1999).
McIlwain, R. B. et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab. Investig. 90, 128–139 (2010).
Fortenberry, J. D. et al. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J. Pediatr. 128, 670–678 (1996).
Dalton, H. J. et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr. Crit. Care Med. 16, 167–174 (2015).
Levi, M. & Van der Poll, T. Inflammation and coagulation. Crit. Care Med. 38, S26–S34 (2010).
Hagberg, H., Gressens, P. & Mallard, C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 71, 444–457 (2012).
Sävman, K., Blennow, M., Gustafson, K., Tarkowski, E. & Hagberg, H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr. Res. 43, 746–751 (1998).
Meso Scale Diagnostics LLC. V-PLEX Human Proinflammatory Panel I (4-PLEX). https://www.mesoscale.com/en/products/v-plex-human-proinflammatory-panel-i-4-plex-k15052d/ (2020).
Meso Scale Diagnostics LLC. R-PLEX Human Serpin E1 (total) Antibody Set. https://www.mesoscale.com/en/products/r-plex-human-serpin-e1-total-antibody-set-f214x/ (2020).
Meso Scale Diagnostics LLC. Human Vascular Injury I Kit. https://www.mesoscale.com/en/products/human-vascular-injury-i-kit-k15135c/ (2020).
Munoz, A. et al. in Risk Assessment and Evaluation of Predictions (eds Lee, M. L. et al.) 3–22 (Springer, New York, NY, 2013).
Ng, D., Antiporta, D., Matheson, M. & Munoz, A. Nonparametric assessment of difference between competing risk hazard ratios: application to racial differences in pediatric chronic kidney disease progression. Clin. Epidemiol. 12, 83–93 (2020).
Dellinger, R. P. Inflammation and coagulation: implications for the septic patient. Clin. Infect. Dis. 36, 1259–1265 (2003).
Risnes, I. et al. Interleukin-6 may predict survival in extracorporeal membrane oxygenation treatment. Perfusion 23, 173–178 (2008).
Wang, S. et al. Laboratory evaluation of hemolysis and systemic inflammatory response in neonatal nonpulsatile and pulsatile extracorporeal life support systems. Artif. Organs 39, 774–781 (2015).
Bhalala, U. S., Koehler, R. C. & Kannan, S. Neuroinflammation and neuroimmune dysregulation after acute hypoxic-ischemic injury of developing brain. Front. Pediatr. 2, 1–12 (2015).
Hirthler, M., Simoni, J. & Dickson, M. Elevated levels of endotoxin, oxygen-derived free radicals, and cytokines during extracorporeal membrane oxygenation. J. Pediatr. Surg. 27, 1199–1202 (1992).
Adrian, K. et al. Cytokine release during long-term extracorporeal circulation in an experimental model. Artif. Organs 22, 859–863 (1998).
Gando, S. Role of fibrinolysis in sepsis. Semin. Thromb. Hemost. https://doi.org/10.1055/s-0033-1334140 (2013).
Vasileiadis, I. et al. Variation of endothelium-related hemostatic factors during sepsis. Microcirculation https://doi.org/10.1111/micc.12500 (2018).
Berner, R. et al. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-α, interleukin (IL)-1β, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr. Res. 44, 469–477 (1998).
Frink, M. et al. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand. J. Trauma. Resusc. Emerg. Med. 17, 49 (2009).
Edinger, F. et al. Comparison of the effect of membrane sizes and fibre arrangements of two membrane oxygenators on the inflammatory response, oxygenation and decarboxylation in a rat model of extracorporeal membrane oxygenation. BMC Cardiovasc. Disord. 20, 294 https://doi.org/10.1186/s12872-020-01581-3 (2020).
Fujii, Y., Tatsumi, E., Nakamura, F. & Oite, T. PaO2 greater than 300 mmHg promotes an inflammatory response during extracorporeal circulation in a rat extracorporeal membrane oxygenation model. J. Thorac. Dis. 12, 749–757 (2020).
Al-Fares, A., Pettenuzzo, T. & Del Sorbo, L. Extracorporeal life support and systemic inflammation. Intensive Care Med. Exp. 7, 46 https://doi.org/10.1186/s40635-019-0249-y (2019).
Tanaka, T., Narazaki, M. & Kishimoto, T. Il-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 https://doi.org/10.1101/cshperspect.a016295 (2014).
Kerr, R., Stirling, D. & Ludlam, C. A. Interleukin 6 and haemostasis. Br. J. Haematol. 115, 3–12 (2001).
Remick, D. G., Bolgos, G. R., Siddiqui, J., Shin, J. & Nemzek, J. A. Six at six: interleukin-6 measured 6 H after the initiation of sepsis predicts mortality over 3 days. Shock 17, 463–467 (2002).
Parameswaran, N. & Patial, S. Tumor necrosis factor-a signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 20, 87–103 (2010).
Bradley, J. R. TNF-mediated inflammatory disease. J. Pathol. 214, 149–160 (2008).
Beshish, A. G. et al. The functional immune response of patients on extracorporeal life support. ASAIO J. 65, 77–83 (2019).
Jackson, S. P., Darbousset, R. & Schoenwaelder, S. M. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 133, 906–918 (2019).
Gando, S. & Otomo, Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit. Care https://doi.org/10.1186/s13054-015-0735-x (2015).
Van De Wouwer, M., Collen, D. & Conway, E. M. Thrombomodulin-protein C-EPCR system integrated to regulate coagulation and inflammation. Arterioscler. Thromb. Vasc. Biol. 24, 1374–1383 (2004).
Chapman, M. P. et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J. Trauma Acute Care Surg. https://doi.org/10.1097/TA.0000000000000885 (2016).
Knottnerus, I. L. et al. Endothelial activation in lacunar stroke subtypes. Stroke 41, 1617–1622 (2010).
van Overbeek, E. C., Staals, J., Knottnerus, I. L., ten Cate, H. & van Oostenbrugge, R. J. Plasma tPA-activity and progression of cerebral white matter hyperintensities in lacunar stroke patients. PLoS ONE 11, e0150740 (2016).
Vila, N., Castillo, J., Dávalos, A. & Chamorro, Á. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31, 2325–2329 (2000).
Vela, J. M., Molina-Holgado, E., Arévalo-Martín, Á., Almazán, G. & Guaza, C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol. Cell. Neurosci. 20, 489–502 (2002).
Yoon, B. H. et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am. J. Obstet. Gynecol. https://doi.org/10.1016/S0002-9378(96)70585-9 (1996).
Tarkowski, E. et al. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke 26, 1393–1398 (1995).
Tarkowski, E. et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin. Exp. Immunol. https://doi.org/10.1046/j.1365-2249.1997.4621483.x (1997).
Yoon, B. H. et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol. https://doi.org/10.1016/S0002-9378(97)70432-0 (1997).
Acknowledgements
The authors would like to acknowledge the two contributing centers as well as the patients described herein and the medical staff who made their complex medical care possible.
Funding
Support for this work included funding from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Awards K23NS076674 and R01NS106292 (MMB).
Author information
Authors and Affiliations
Contributions
M.M.B. conceived and designed the study. S.D.C., A.T., R.J.F., C.F.S., A.D.E., J.M.S. and M.M.B. all contributed to the acquisition of data. D.K.N. and M.K.C. provided statistical analysis and interpretation of the data. S.D.C. and M.M.B. drafted the article while all authors contributed to the revision and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from parents or legal guardians within 24 h of ECMO initiation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Caprarola, S.D., Ng, D.K., Carroll, M.K. et al. Pediatric ECMO: unfavorable outcomes are associated with inflammation and endothelial activation. Pediatr Res 92, 549–556 (2022). https://doi.org/10.1038/s41390-021-01817-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01817-8
This article is cited by
-
Neuromonitoring During ECMO Support in Children
Neurocritical Care (2023)
-
Stroke in pediatric ECMO: a target for prevention and improvement
Pediatric Research (2022)