Abstract
Background
Conventional sampling for pharmacokinetic clinical studies requires removal of large blood volumes from patients. This can result in a physiological/emotional burden for children. Microsampling to support pharmacokinetic clinical studies in pediatrics may reduce this burden.
Methods
Parents/guardians and bedside nurses completed a questionnaire describing their perception of the use of microsampling compared to conventional sampling to collect blood samples, based on their child’s participation or their own role within a paired-sample pharmacokinetic clinical study. Responses were based on a seven-point Likert scale and were analyzed using frequency distributions.
Results
Fifty-one parents/guardians and seven bedside nurses completed a questionnaire. Parents/guardians (96%) and bedside nurses (100%) indicated that microsampling was highly acceptable and recommended as a method for collecting blood samples for pediatric patients. Responding to a question about the child indicating pain during the blood sampling procedure, 61% of parent/guardians reported no pain in their children, 14% remained neutral, and 26% reported that their child indicated pain; 71% of the bedside nurses slightly agreed that the children indicated pain.
Conclusions
This study strongly suggests that parents/guardians and bedside nurses prefer microsampling to conventional sampling to conduct pediatric pharmacokinetic clinical studies. Employing microsampling may support increased participation by children in these studies.
Impact
-
Pharmacokinetic clinical studies require the withdrawal of blood samples at multiple times during a dosing interval. This can result in a physiological or emotional burden, particularly for neonates or pediatric patients.
-
Microsampling offers an important opportunity for pharmacokinetic clinical studies in vulnerable patient populations, where smaller sample volumes can be collected. However, microsampling is not commonly used in clinical studies.
-
Understanding the perceptions of parents/guardians and bedside nurses about microsampling may ascertain if this technique offers an improvement to conventional blood sample collection to perform pharmacokinetic clinical studies for pediatric patients.
Similar content being viewed by others
Introduction
Pharmacokinetic clinical studies are performed to establish an understanding of the dose–concentration relationship of a drug. From these studies, optimal dosing can be established. These studies are performed during the registration process of a new drug and post registration in special patient populations. Pharmacokinetic clinical studies require the withdrawal of blood samples at multiple times during a dosing interval from a group of healthy volunteers or patients. Relatively large cumulative volumes of blood, typically between 0.5 and 2 mL per sample, are taken using either an arteriovenous cannula or venous phlebotomy.1 This conventional large volume approach to sampling can be problematic for pediatric patients as the frequency and volume of withdrawn blood is not always easily obtainable. Blood sampling can inflict pain, can cause distress, and, particularly in critically ill patients, can also cause iatrogenic anemia.2,3,4
Recent advances in technology have led to improvements in assay sensitivity and the ability to measure drug concentrations in smaller volumes of biological fluids. Microsampling techniques take advantage of this advance to enable the collection of smaller blood samples (50 µL or less) from a skin prick rather than via venous phlebotomy or using an indwelling cannula.
Microsampling is being implemented for a range of clinical studies, including drug development and pre-clinical studies,5,6,7 pharmacokinetic and toxicokinetic studies,7,8,9,10 and clinical bridging studies.11 Despite the increasing use of microsampling to support clinical studies, the clinical application, especially in pharmacokinetics, in pediatric patients remains limited.
Parents/guardians may refuse involvement of their child in any clinical research study. By understanding the perceptions of parents/guardians about microsampling, we may ascertain if this technique offers an improvement to conventional blood sample collection for pediatric patients. Pediatric bedside nurses are familiar with microsampling, in terms of the collection of capillary samples for routine blood gas analysis. Some studies have used microsampling in pediatrics, such as volumetric absorptive microsampling as a tool for self-sampling collection to monitor hemoglobin A1c in diabetic patients,12 while others have used dried blood spots to (i) self-collect samples at home for therapeutic drug monitoring of antiepileptic drugs in children with epilepsy13 and (ii) to investigate the relationship between pharmacokinetic parameters, side effects and effectiveness of risperidone in children with autism, and severe behavioral problems.14 Other larger studies performed in adults have found microsampling to be acceptable for therapeutic drug monitoring,15,16,17 toxicology,18 and observational clinical studies.19
Nested within a pharmacokinetic clinical study in a Pediatric Intensive Care Unit (PICU; HREC/17/QRCH/45) paired plasma samples were collected (1) by skin prick as a capillary microsample, and (2) by arterial venous line for conventional sampling. However, for a microsample to be successfully used in clinical practice, the acceptability of the collection of blood samples using microsampling needs to be understood. Therefore, the aim of this survey was to evaluate the perceptions of parents/guardians and bedside nurses about the use of microsampling during the pharmacokinetic clinical study as an alternative sampling technique to facilitate clinical studies in pediatric patients in general.
Methods
Study design
A survey nested within a paired-sample pharmacokinetic clinical study, involving conventional sampling and microsampling, was conducted in the PICU at the Queensland Children’s Hospital in Brisbane, Australia. The survey, containing two independently administered questionnaires, was approved by the Human Research Ethics Committee of the Queensland Children’s Hospital (HREC/17/QRCH/45).
Clinical staff training
Bedside nurses were trained on the overall functionality and handling of two types of microsampling devices selected for the clinical study. These included capillary microsampling, where <50 µL of whole blood obtained by a skin prick is collected into a heparinized tube, and microfluidic capillary tube sampling (Microsampling Wing™, ref. 20), in which ~23 µL of whole blood obtained by a skin prick is collected into a micro-channel containing anticoagulant. The research staff were provided with direct demonstrations and information sheets for collection procedures using the microfluidic capillary sampling device and capillary microsampling. Clinical bedside PICU staff are familiar with capillary microsampling as a common technique for obtaining clinical blood samples from hospitalized children.
Procedure
Data were collected between March 2019 and August 2020. Critically ill children receiving the study drug, as prescribed by the treating physician, and admitted to the PICU of the Queensland Children’s Hospital in Brisbane, Australia, were eligible for the pharmacokinetic clinical study. Prior to sampling, written informed consent was obtained from the parent/guardian. Paired whole-blood samples were simultaneously collected at five pre-defined time points: prior to administration of the study drug and then four times post administration. The sample collection process produced two sets of liquid plasma samples: one microsample and one conventional sample. For capillary microsamples, the patient’s finger was cleaned with alcohol and the skin-prick puncture was performed using a lancet device (either Haemolance Plus®, low flow 25 G × 1.4 mm or BD Microtainer Quikheel Infant Lancet, 1 mm × 2.5 mm).
Parents/guardians of patients in the PICU, who agreed to participate in the pharmacokinetic clinical study, were asked to complete questionnaire #1 after their children were enrolled and participated in the study. Along with the questionnaire, information sheets were provided that explained the use of microsampling techniques to facilitate the collection of samples from a patient to support clinical studies.
The bedside nurse caring for the study patient was asked to complete questionnaire #2 after completion of blood sampling. All questionnaires were anonymous.
Questionnaire #1 consisted of 12 questions and questionnaire #2 had 9 questions. The responses were based on a seven-point Likert scale (1 = strongly agree, 2 = agree, 3 = slightly agree, 4 = neutral, 5 = slightly disagree, 6 = disagree, and 7 = strongly disagree). The surveys were designed by the research staff to measure overall perceptions of parents/guardians and bedside nurses about the use of microsampling techniques to conduct pharmacokinetic clinical studies in pediatric patients.
Description of answers
Ordinal data collected from the questionnaires were analyzed using frequency and frequency distributions with data presented as percentages. The responses from the questionnaires were de-identified prior to data analysis.
Results
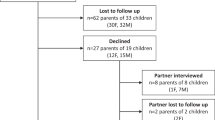
Eighty-six parents/guardians consented for their children (age range: 1 month–12 years) to participate in the pharmacokinetic clinical study. Of these, 51 parents/guardians completed questionnaire #1. The results of the questionnaire #1 are summarized in Supplementary material S1. The demographic characteristics of the pediatric patients are shown in Table 1.
Responses were collected from seven bedside nurses (questionnaire #2). Four of the nurses collected microsamples from critically ill pediatric patients using capillary microsampling and three nurses collected microsamples from critically ill pediatric patients using microfluidic capillary devices. The results of the questionnaire #2 are shown in Supplementary material S2.
Parents/guardians reported whether they had concerns regarding the use of microsampling techniques before their children participated in the clinical pharmacokinetic study. Only 6% (n = 3/51) of the respondents slightly agreed that they had concerns. The majority (98%, n = 50/51) of the parents/guardians reported confidence in the bedside nurses performing the skin-prick procedure and 100% (n = 51/51) of the respondents agreed or strongly agreed that they fully understood the implications of being part of the pharmacokinetic clinical study using microsampling techniques, including the amount of blood required to be withdrawn from their children. All of the bedside nurses (100%, n = 7/7) agreed or strongly agreed that they were confident in the training and their use of microsampling techniques required to conduct the clinical study.
Overall, 94% (n = 48/51) of the parents/guardians and 100% (n = 7/7) of the bedside nurses perceived that microsampling techniques are less invasive and less painful than conventional blood sampling. Furthermore, 90% (n = 46/51) of parents/guardians and 100% (n = 7/7) of bedside nurses agreed, slightly agreed, or strongly agreed that they would recommend microsampling to support clinical studies in pediatric patients, while 10% (n = 5/51) of the parents/guardians remained neutral (Fig. 1). There were mixed responses from parents/guardians and bedside nurses on how they perceived the pain indicated by the child during the skin-prick sampling procedure (Fig. 1). Sixty-one percent (n = 31/51) of the parents/guardians disagreed, slightly disagreed, or strongly disagreed that their children indicated pain. However, the skin-prick procedure is not always without pain, 14% (n = 7/51) of the respondents remained neutral, and 26% (n = 13/51) agreed, slightly agreed, or strongly agreed that their children indicated pain during the procedure. The majority (71%, n = 5/7) of the bedside nurses only slightly agreed that the child indicated pain when collecting the sample from the skin prick using microsampling devices, while 29% (n = 2/7) only slightly disagreed with this statement. In a section provided in the questionnaire for additional comments, two parents/guardians commented that they observed discomfort rather than pain in their children due to the skin-prick procedure.
Parents/guardians and bedside nurses report on three questions: “I would recommend the use of microsampling techniques to perform clinical studies in sick children to other parents and guardians,” “I believe microsampling techniques are less invasive and less painful,” “The child indicated pain during the sampling procedure.” Reponses were analyzed using frequency and frequency distributions with data presented as percentages.
Another important feature evaluated in this survey was the perception of parents/guardians regarding their children’s overall needs being met during the pharmacokinetic clinical study. The majority of the parents/guardians (92%, n = 47/51) agreed that their child’s needs were met, 4% (n = 2/51) of the parents/guardians disagreed or strongly disagreed with this statement, while 4% (n = 2/51) remained neutral. In the additional comments section, one parent commented on the clinical staff prioritizing the clinical study over the medical procedures.
Additional parent/guardian comments are provided in Supplementary material S1.
Discussion
We have investigated the overall perceptions of parents/guardians and bedside nurses about the use of microsampling as an alternative sampling technique to facilitate pharmacokinetic clinical studies in pediatric patients.
There are physiological concerns regarding the impact on pediatric patients based on the amount of blood required to participate in a pharmacokinetic study. This requires consideration of the sampling frequency and the sample volume needed to accurately describe drug concentrations.21, 22 This problem is more evident in young children due to their relatively small circulatory blood volume.22 Large amounts of blood sampling can cause iatrogenic anemia, compromise volume circulation, and lead to the need for a blood transfusion.1 Conventional venous blood sampling using an indwelling catheter requires the removal of additional ‘discard’ volumes of blood (up to 2–5 mL per sample). This adds a risk of thrombosis and infection of the cannula and requires additional intravenous flushes to prevent blood clotting and blocking of the intravenous cannula. The guidelines recommend that the amount of blood sampling in young infants should not exceed 3% of the total blood volume in a 4-week period and 1% at any single time.23 These guidelines can limit the number of blood samples that can be collected for participation in a pharmacokinetic clinical study.
As an alternative blood sampling technique, microsampling can be collected from a skin prick. Skin-prick collection can be associated with distress and discomfort or some level of pain.24, 25 During the pharmacokinetic clinical study, it was observed that two older pediatric patients (age range 5–10 years) refused the skin-prick procedure after the first microsample was taken due to distress associated with the skin prick. As a result, subsequent microsamples were not collected from these patients. In addition to affecting pediatric patients, child’s pain and distress during needle procedures may cause anxiety-related reactions in parents/guardians and bedside nurses.26 In our study, ~75% of bedside nurses and 40% of parents/guardians completing the questionnaire perceived that the child experienced pain (with some parents noting that they perceived this as discomfort) during the microsampling procedure. In a section provided in the questionnaire for additional comments, parents/guardians commented that discomfort rather than pain was observed in their children due to the skin-prick procedure. A study by Fradet et al.24 reported that considerably more distress behaviors were observed in pediatric patients (aged 3–17 years) undertaking blood-related venipuncture procedures compared with those patients experiencing a skin prick. This is more evident in the PICU, where the majority of the patients experience phlebotomy at the time of admission either via venipuncture or by inserting an intravenous cannula to collect blood samples as part of their routine clinical care.
It is of interest that the perception of over half of the parents/guardians completing the questionnaire was that their children were unaffected by the skin prick used to collect the microsample during the pharmacokinetic clinical study. In addition, the results from the questionnaires of both parents/guardians and bedside nurses showed high acceptability of microsampling as an alternative method for blood sampling.
Although the questionnaire for the bedside nurses did not specify the type of microsampling technique used in the study, we documented whether capillary microsampling or microfluidic capillary sampling were used to collect the sample from the patient. The results of the survey showed that both of the microsampling techniques used were acceptable to the nursing staff for use to support clinical studies in pediatric settings.
It is important to note that the sample size of the responses may serve as a limitation in our study. This is particularly the case for the bedside nurses’ survey, in which only seven questionnaires were collected from the nurses caring for the study participants. The PICU wards provide specialized and advanced treatments to critically ill patients, who constantly requires high levels of monitoring from bedside nurses. This may have limited the collection of additional questionnaires from the nurses.
We did not prospectively evaluate the overall perceptions of parents/guardians prior to the study. We conducted the questionnaire survey after the microsampling techniques were used in the pharmacokinetic clinical study. This limited our ability to understand parents/guardians’ initial thoughts concerning the participation of their children in a study that involved microsampling techniques to support clinical studies.
This study identified the perceptions from both parents/guardians and bedside nurses about the use of microsampling when collecting clinical samples from pediatric patients. The information gathered in these surveys showed that microsampling has a high level of acceptability as a method for collecting blood samples for pediatric patients. Furthermore, microsampling would be recommended as a blood sampling technique by both parents/guardians and bedside nurses.
References
Altamimi, M., Choonara, I. & Sammons, H. Invasiveness of pharmacokinetic studies in children: a systematic review. BMJ 6, e010484 (2016).
Lister, P., Peters, M. & Petros, A. Effects of blood sample volume on hematocrit in critically ill children and neonates. Pediatr. Anesthesia 18, 420–425 (2008).
Howie, S. R. Blood sample volumes in child health research: review of safe limits. Bull. World Health Organ. 89, 46–53 (2011).
Dorofaeff, T. et al. Uncertainty in antibiotic dosing in critically ill neonate and pediatric patients: can microsampling provide the answers? Clin. Ther. 38, 1961–1975 (2016).
Dillen, L. et al. Blood microsampling using capillaries for drug-exposure determination in early preclinical studies: a beneficial strategy to reduce blood sample volumes. Bioanalysis 6, 293–306 (2014).
Raynaud, F. et al. Capillary microsampling of mouse blood in early pre-clinical studies: a preferred alternative to dried blood spot sampling. J. Bioanal. Biomed. 8, 028–033 (2016).
Jonsson, O. et al. Capillary microsampling of 25 µl blood for the determination of toxicokinetic parameters in regulatory studies in animals. Bioanalysis 4, 661–674 (2012).
Verhaeghe, T. et al. The application of capillary microsampling in GLP toxicology studies. Bioanalysis 9, 531–540 (2017).
Korfmacher, W. et al. Capillary microsampling of whole blood for mouse PK studies: an easy route to serial blood sampling. Bioanalysis 7, 449–461 (2015).
Korfmacher, W. et al. Utility of capillary microsampling for rat pharmacokinetic studies: comparison of tail-vein bleed to jugular vein cannula sampling. J. Pharmacol. Toxicol. Methods 76, 7–14 (2015).
Valero, Y. C. G. et al. Analysis of capillary microsamples obtained from a skin-prick to measure vancomycin concentrations as a valid alternative to conventional sampling: a bridging study. J. Pharm. Biomed. Anal. 169, 288–292 (2019).
Verougstraete, N. et al. Volumetric absorptive microsampling at home as an alternative tool for the monitoring of HbA1c in diabetes patients. Clin. Chem. Lab. Med. 55, 462–469 (2017).
Linder, C. et al. Parents’ perspectives on dried blood spot self‐sampling from children with epilepsy: a mixed‐method study. Acta Paediatr. 109, 2789–2798 (2020).
Kloosterboer, S. M. et al. Risperidone plasma concentrations are associated with side effects and effectiveness in children and adolescents with autism spectrum disorder. Br. J. Clin. Pharmacol. 87, 1069–1081 (2020).
Kloosterboer, S. M. et al. Dried blood spot analysis for therapeutic drug monitoring of antipsychotics: drawbacks of its clinical application. Ther. Drug Monit. 40, 344–350 (2018).
Veenhof, H. et al. Effects, costs and implementation of monitoring kidney transplant patients’ tacrolimus levels with dried blood spot sampling: a randomized controlled hybrid implementation trial. Br. J. Clin. Pharmacol. 86, 1357–1366 (2020).
Scuderi, C. E. et al. Kidney transplant recipient’s perceptions of blood testing through microsampling and venepuncture. Bioanalysis 12, 873–881 (2020).
Van Uytfanghe, K., Heughebaert, L. & Stove, C. P. Self-sampling at home using volumetric absorptive microsampling: coupling analytical evaluation to volunteers’ perception in the context of a large scale study. Clin. Chem. Lab. Med. 1 e185–e187 (2020).
Boons, C. C. et al. Feasibility of and patients’ perspective on nilotinib dried blood spot self-sampling. Eur. J. Clin. Pharmacol. 75, 825–829 (2019).
Shimadzu. Microsampling Wing Device https://www.shimadzu.com/opt/products/msw2/o-od0gjn0000008nt8.html (2018).
Kern, S. E. Challenges in conducting clinical trials in children: approaches for improving performance. Expert Rev. Clin. Pharmacol. 2, 609–617 (2009).
Patel, P. et al. Facilitating pharmacokinetic studies in children: a new use of dried blood spots. Arch. Dis. Child. 95, 484–487 (2010).
European Medicines Agency (EMA). Guidelines on the Investigation of Medicinal Products in the Term and Preterm Neonate (European Medicines Agency, 2007).
Fradet, C. et al. A prospective survey of reactions to blood tests by children and adolescents. Pain 40, 53–60 (1990).
Kennedy, R. M., Luhmann, J. & Zempsky, W. T. Clinical implications of unmanaged ne–edle-insertion pain and distress in children. Pediatrics 122(Suppl. 3), S130–S133 (2008).
Smith, R. W. et al. Caregivers’ responses to pain in their children in the emergency department. Arch. Pediatr. Adolesc. Med. 161, 578–582 (2007).
Acknowledgements
We would like to acknowledge the parents/guardians who completed the survey, the children who participated in the pharmacokinetic clinical study, and the clinical staff members of the PICU at the Queensland Children’s Hospital, Brisbane, Australia, for completing the survey and for their support and assistance with sample collection and other relevant tasks for this study. Y.G.V. is a recipient of a Research Training Scholarship from The University of Queensland. S.L.P. is a recipient of an Early Career Research Fellowship from the Australian National Health and Medical Research Council (APP1142757). J.A.R. is a recipient of an Australian National Health and Medical Research Council Fellowship (APP1048652).
Author information
Authors and Affiliations
Contributions
S.L.P., Y.G.V., and J.A.R. conceptualized and designed the study; T.D. participated in designing the study. T.D and L.P. participated in the acquisition of data. S.L.P., L.P., and Y.G.V. participated in the analysis and interpretation of data. Y.G.V. and S.L.P. drafted the manuscript. All authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The study protocol was approved by the local Institutional Review Board and Ethics Committee. Parents/guardians of all critically ill pediatric patients gave informed consent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Guerra Valero, Y., Dorofaeff, T., Parker, L. et al. Microsampling to support pharmacokinetic clinical studies in pediatrics. Pediatr Res 91, 1557–1561 (2022). https://doi.org/10.1038/s41390-021-01586-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01586-4