Abstract
Background
Enriched language exposure may benefit infants in the neonatal intensive care unit. We hypothesized that changes in neonatal electroencephalogram (EEG) coherence during sleep, in response to maternal voice exposure, predict language development.
Methods
Convalescent neonates underwent 12-h polysomnography. A recording of the mother’s voice was randomized to continuous playback in the first or second 6 h. We calculated the imaginary coherence (ICOH—a measure of functional connectivity) between EEG leads. Spearman correlations were computed between ICOH and 18-month Bayley-III language scores.
Results
Thirty-five neonates were included (N = 18 33-to-<35 weeks gestation; N = 17 ≥ 35 weeks). Predictive value of ICOH during neonatal non-rapid eye movement (NREM) sleep was left lateralized, and varied with gestational age and voice playback. ICOH in the left-hemispheric (C3-Cz; T3-Cz) channels across multiple EEG frequency bands was associated with 18-month language scores (rho = −0.34 to −0.48). The association was driven by neonates born at 33–34 weeks gestation, and a trend suggested a possible effect of maternal voice at some EEG frequencies. Right hemisphere ICOH (C4-Cz; T4-Cz) was not associated with language outcome.
Conclusions
Left-hemispheric EEG functional connectivity during neonatal NREM sleep shows early signs of physiologic asymmetry that may predict language development. We speculate that sleep analyses could have unique prognostic value.
Impact
-
During neonatal NREM sleep, EEG functional connectivity predicts future language development.
-
Left temporal and central EEG coherence—specifically the imaginary component of coherence—is predictive, whereas the same analysis from the right hemisphere is not.
-
These results appear to vary according to the infant’s gestational age, and a trend suggests they may be enhanced by measuring functional connectivity during exposure to the mother’s voice.
-
These findings identify early evidence of physiologic differentiation within the cerebral hemispheres and raise the possibility that neonatal NREM sleep has a role to play in language development.
Similar content being viewed by others
Introduction
During the first half of the third trimester, important cerebellar and white matter growth occurs, followed at 34–40 weeks postmenstrual age by a rapid acceleration of cortical gray matter development.1 During these critical periods of brain development, neonates who require intensive care are exposed to a hospital environment.
Many modern neonatal intensive care units (NICUs) now provide care for individual infants in private rooms. While clinical and architectural endeavors target reductions in ambient noise in the NICU,2,3 the impact of noise reduction on sick infants remains unclear. A quiet environment may provide increased opportunity for sleep, but decreased language exposure in the private room could have adverse consequences.4
Animal models demonstrate the time-specific role of the acoustic environment during auditory cortex organization and, suggest that sound deprivation can result in abnormal structure and function of the auditory system.5,6,7 Conversely, animal data also suggest that the auditory environment enrichment may have beneficial effects on neuronal development.8 A study of human infants born at <30 weeks gestation demonstrated that abnormalities of the nonprimary auditory cortex, demonstrated on serial brain MRI, were associated with lower language scores by age 2 years.9
We hypothesized that enriched language exposure through playback of maternal voice recordings may benefit infants in the NICU. Our initial data demonstrated that neonates born at ≥35 weeks, but not those born at 33–34 weeks, had increased wakefulness in response to their mother’s voice, and also that exposure to the mother’s voice during sleep protected against awakening after bursts of loud noises.10 Moreover, our previous work demonstrated that quantitative sleep and electroencephalogram (EEG) markers shortly after birth may have prognostic value, in that they can show associations with 18-month neurodevelopmental outcomes.11 Others have suggested that neonatal EEG coherence—a measure of functional connectivity—reflects brain maturity,12,13 and that there are measurable differences in neonatal EEG coherence between infants with hypoxic-ischemic encephalopathy and controls.14 Initial studies of developmentally focused NICU care suggest that methods of NICU care delivery can alter EEG coherence among preterm infants, and that neurophysiological effects may persist in childhood.15,16 Thus, we hypothesized that neonatal EEG coherence changes in response to maternal voice may predict neurodevelopmental outcomes, especially in the language domain.
Methods
This study was approved by a Michigan Medicine Institutional Review Board. A parent of every enrolled infant provided written informed consent.
Subjects
Late-preterm and term newborns admitted to a level IV single-patient room NICU were recruited for participation in this study. Inclusion criteria were birth at ≥33 weeks to 41 weeks gestation, need for NICU admission, and stable vital signs in an open crib. Exclusion criteria were congenital brain abnormalities, airway abnormalities that were likely to cause sleep-disordered breathing, suspected or known genetic abnormalities that would alter developmental trajectories, severe encephalopathy that altered sleep–wake cycling, and abnormal neonatal hearing screen (brainstem auditory evoked responses). The clinical and demographic profile of included infants were presented elsewhere,10 though the 18-month outcomes have not been presented previously. Our previous work delineated a clear difference in response to maternal voice recording for infants born at 33–34 weeks gestation compared with infants born at ≥35 weeks gestation.10 Thus, we planned the present analyses to compare these two age groups.
Measurements
Every infant underwent a 12-h polysomnogram at the bedside in the NICU. Recordings were generally started after the infant’s evening feed (~8 p.m.). A registered polysomnographic technologist experienced with pediatric studies remained at the bedside throughout the study, to ensure high-quality recordings. Details of the polysomnographic recording procedures and scoring have been presented elsewhere.10 The EEG leads were placed according to the international 10–20 system, modified for neonates17,18 and EEG was sampled at 256 Hz. Clinical care and feeding regimens were maintained during the polysomnogram. A continuous voice playback of the infant’s mother reading children’s books19,20 was randomized 1:1 to be played for the first or second 6-h segment of the polysomnogram. The playback device was placed on a bedside table 1–2 feet from the infant.
All infants were invited to return for follow-up neurodevelopmental assessments with Bayley-III and neurological examinations at 18 months corrected age. The developmental outcome examiners were blinded to the advanced EEG analysis results.
Analysis
We calculated Spearman correlations between clinical and polysomnographic variables and Bayley-III language scores. We then computed complex coherence between the EEG leads over sequential 3-second time epochs throughout the polysomnograms. Our previous work suggested that quantitative features of non-rapid eye movement (NREM, previously referred to as quiet) sleep were most predictive of outcomes among sick newborns.11 Thus, we focused our analyses on NREM sleep epochs; other sleep and wake stages were analyzed secondarily. Specifically, we evaluated mean coherence between electrode pairs. As we hypothesized that coherence associated with language exposure would be lateralized, we focused our analyses on the central and temporal electrode pairs (C3-Cz and C4-Cz; T3-Cz and T4-Cz). Central electrodes are typically available in clinical polysomnography; as language function is primarily localized to the temporal lobe, we also analyzed data from the temporal electrodes. We did not employ artifact reduction, nor did we omit segments with poor signal quality. However, to render analyses generalizable to other NICUs, we relied on polysomnogram-defined sleep–wake stages for our analyses.
The coherence calculation used Welch’s method to reduce the variance of the estimates.21 We used the imaginary part of the coherence (ICOH) as a metric to mitigate the impact of volume conduction between leads.22 To examine the effect of maternal voice playback and sleep stage, we identified the associated time epochs. A bootstrap estimate of the mean ICOH for each lead pair was computed for the specified frequency range, sleep stage, and playback condition for each participant.
To evaluate associations with 18-month outcomes, we computed Spearman correlations between mean ICOH and Bayley-III scores. To evaluate the difference in Spearman correlations during versus without maternal voice playback, we calculated 10,000 bootstrap samples of the original data by sampling the ICOH and Bayley language data with replacement. For each sampling, the Spearman correlation was computed with and without maternal voice playback, and the difference of the correlation coefficients was computed. This procedure produced a bootstrap distribution for each correlation coefficient and the difference of the correlation coefficients such that the null hypothesis was rejected, if zero was outside the 95% confidence interval of the distribution.
All analyses were conducted using Matlab (MathWorks, Natick, MA) and statistical significance was defined as p value <0.05. The priority in this novel application of coherence analysis to neonatal sleep was to identify associations that may provide insight into neonatal sleep physiology. For this reason, we did not adjust for multiple comparisons.
Results
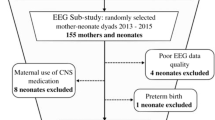
Forty-seven infants were enrolled, 1 was later excluded due to a genetic diagnosis associated with developmental delay, and 35 returned for 18-month follow-up (N = 18 at 33-to-<35 weeks gestation, N = 17 at ≥35 weeks). Three did not have maternal voice playback exposure so were excluded from part of the analysis. Clinical and demographic data are presented in Table 1.
Bayley-III scores were, on average, ~5 points (1/3 of a standard deviation) lower for the language subscale than for cognitive and motor subscales (97.5 ± 4.4, compared to 102.6 ± 7.1 and 103.5 ± 2.1). None of the clinical or standard polysomnographic variables listed in Table 1, from the neonatal period, were associated with 18-month Bayley-III scores (p > 0.15 for each association).
The predictive value of ICOH during neonatal NREM sleep was lateralized, and varied with gestational age. ICOH in the left-hemispheric channels across multiple EEG frequency bands was associated with 18-month language scores (C3-Cz: rho = −0.15 to −0.43, p < 0.05; T3-Cz: rho = 0.08 to −0.49, p < 0.05; Fig. 1). However, right hemisphere ICOH (C4-Cz and T4-Cz) was not associated with language outcome (Table 2). The association for left hemisphere ICOH was isolated to neonates born at <35 weeks gestation.
Among 35 neonates, the imaginary component of the coherence (ICOH) between left central and temporal EEG electrodes (C3-Cz and T3-Cz) was predictive of 18-month language outcome, especially during playback of a maternal voice recording (a, c, e, g). There were no such associations of right central and temporal (C4-Cz and T4-Cz) ICOH and outcome, with or without maternal voice playback (b, d, f, h).
In contrast, the associations between EEG ICOH and Bayley-III scores during REM sleep, indeterminate sleep, or wakefulness were less consistent, though still left lateralized (supplemental table). There was no association between EEG ICOH in these central and temporal channels, and the Bayley-III cognitive or motor scale scores.
The bootstrap distributions of the difference of the correlation coefficients with and without voice playback for the T3-Cz electrode pair are displayed in Fig. 2. The correlation coefficients were most different at the higher frequency bands, while they did not appear to change in response to maternal voice playback at the lower frequencies.
Histograms from the bootstrap analyses of the difference in Spearman correlation of the imaginary component of the coherence (ICOH) during NREM sleep, using the T3-Cz electrode pair, and Bayley-III language scores, with and without maternal voice playback, for 32 infants (a: 4 to <8 Hz; b: 8 to <12 Hz; c: 12 to <16 Hz; d: 16–30 Hz). The vertical blue lines indicate the 2.5 and 97.5 percentile values. Differences in ICOH with and without maternal voice playback were greater for higher EEG frequencies.
Discussion
Results of this cross-sectional polysomnographic study of 35 neonates in an intensive care unit suggest that patterns of EEG functional connectivity during NREM sleep show left-lateralized predictive value for language development at 18 months. These results were distinct from those for 18-month cognitive and motor outcomes, which showed no associations with EEG functional connectivity at these electrode positions, and therefore suggest plausible underlying neuroanatomic specificity, as opposed to spurious results. Functional connectivity at certain frequencies was most predictive in the subset of infants born at 33-to-<35 weeks gestation. In addition, a trend suggested that for some frequencies, playback of the mother’s voice may enhance the predictive value of the concurrent ICOH for language outcomes. In the absence of strong prognostic value from traditional neonatal clinical data, neurologic exam scores, or standard polysomnographic variables, we speculate that digital analyses of the sleep EEG could have unique value.
Language development is a complex and dynamic process that is vulnerable to disruption during the time that most sick neonates are cared for in the NICU. Among 90 very preterm infants (<30 weeks) without severe brain injury who were studied with serial brain MRI and 2-year follow-up, disturbance of the maturation of nonprimary cortex (but not primary cortex) was associated with abnormal language development.9 A landmark report suggested that for preterm infants, the private NICU room environment is associated with reduction of expected sulcal asymmetry in the auditory cortex by term equivalent age, and that this manifests as poorer language development at age 2 years.4 However, others reported that there was no discernible difference in white matter diffusion parameters between infants cared for in private rooms versus an open bay NICU, and that these MRI parameters did not predict 2-year language scores.9 Here, we demonstrate that a functional measure of brain development—the EEG ICOH—may also measure relevant brain maturation that relates to the language development for some preterm infants. We also add that this measure may change with enriched exposure to the maternal voice.
Others have reported decreased heart rate and respiratory rate for preterm infants (30 to <37 weeks gestation) during exposure to recorded lullabies and taped maternal voice, with an effect that was most pronounced for infants with higher gestational ages.23 For the present cohort, exposure to the maternal voice playback insulated neonates from arousals associated with peak NICU noise epochs. The impact of voice exposure on immediate polysomnographic data was highest in late-preterm and term infants.10 Yet, the present analyses raise the possibility that functional connectivity changes in response to the same voice exposure may predict language outcomes for preterm infants rather than late-preterm and term infants. The impact of maternal voice exposure may not be isolated to late-preterm and term neonates, but instead may differ across stages of brain maturation. Others have reported lateralized maturational EEG changes in a nonlinear spectral measure for preterm neonates who were offered skin to skin contact over 8 weeks.24 Such findings, combined with those presented here, underscore that specialization of the brain already exists in preterm infants—responses to tactile sensory stimuli localize to the right hemisphere, while auditory stimuli localize to the left hemisphere.
Importantly, auditory stimulation is but one of multiple sensory modalities that influence brain development. All neonates, even those who appear healthy after birth, express age-specific consequences of brain maturation related to maternal-placental-fetal factors. This is particularly a consideration for preterm neonates—even late-preterm infants—and their immature brain maturation at birth. There remains a gap in developmental care research with regard to individual infants’ responses to interventions relative to genetic vulnerability or resilience and adversities that are not detectable by contemporary maternal care and surveillance. These variables may alter infants’ responses to a particular enrichment paradigm (such as sound or tactile input) according to the effect on the cortical region required for the response.
We report results from 35 neonates who underwent gold-standard bedside polysomnography and 18-month developmental follow-up. Our study focused on detailed analysis of each newborn’s sleep physiology; the sample size was, by necessity, limited. In this novel application of coherence analyses in neonates, we prioritized identification of new insight into sleep physiology and neurodevelopment, and did not adjust for multiple comparisons. Results therefore merit replication. Some additional limitations warrant discussion. We analyzed the potential impact of changes in the acoustic environment on neonatal EEG-sleep data, but we acknowledge that other factors also influence sleep in the NICU (e.g., handling,25 medications, and light exposure). We are unable to account for these other factors in the present analysis. We did not measure exposure to live spoken words in the home prior to conducting the Bayley-III exams. The home language environment, and other factors, may have influenced the developmental trajectories of participants in this study. Future work should include more comprehensive evaluation and statistical adjustment for socioeconomic status and other factors that may relate to developmental outcomes; such measures were beyond the scope of this initial study.
Conclusions
Clearly predictive, noninvasive measures of neonatal brain development are of utmost importance to clinicians and parents alike. We demonstrate for the first time that lateralized neonatal EEG coherence during quiet sleep is associated with 18-month language development. Whether coherence is a fixed feature (e.g., reflects a subtle, unchangeable functional brain abnormality) or could be improved with enriched language exposure and careful attention to early language development remains an open question. Importantly, our findings were most robust for the youngest neonates in this study. Language exposure interventions likely need to be tailored to infants’ gestational and postmenstrual age. We have no clear explanation for why impact of the maternal voice might be more rather than less prominent in a window that lies well before the end of fetal development. We speculate however that such a finding, if confirmed, could have implications for language exposure in utero, not just after premature delivery. The optimal timing of language exposure, the influence of that exposure on sleep, and the association with developmental trajectory should be key priorities for continued research.
References
Matthews, L. G. et al. Brain growth in the NICU: critical periods of tissue-specific expansion. Pediatr. Res. 83, 976–981 (2018).
White, R. D. Designing environments for developmental care. Clin. Perinatol. 38, 745–749 (2011).
White, R. D. Recommended standards for newborn ICU design, 9th edition. J. Perinatol. 40, 2–4 (2020) .
Pineda, R. G. et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit envrionments. J. Pediatr. 164, 52–60 (2014).
de Villers-Sidani, E., Chang, E. F., Bao, S. & Merzenich, M. M. Critcal period window for spetral tuning defned in the primay auditory cortex (A1) in the rat. J. Neurosci. 27, 180–189 (2007).
Mowery, T. M., Kotak, V. C. & Sanes, D. H. Transient hearing loss within a critical period causes persistent changes to cellular properties in adult auditory cortex. Cereb. Cortex 25, 2083–2094 (2015).
Oliver, D. L., Izquierdo, M. A. & Malmierca, M. S. Persistent effects of early augmented acoustic environment on the auditory brainstem. Neuroscience 184, 75–87 (2011).
Bose, M. et al. Effect of the environment on the dendritic morphology of the rat auditory cortex. Synapase 64, 97–110 (2010).
Monson, B. B. et al. Differential rates of perinatal maturation of human primary and nonprimary auditory cortex. eNeuro 5 e0380-17.2017 1–12 (2018).
Shellhaas, R. A., Burns, J. W., Barks, J. D. E., Hassan, F. & Chervin, R. D. Maternal voice and infant sleep in the neonatal intensive care unit. Pediatrics 144 (2019).
Shellhaas, R. A. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep 40, zsx144 (2017).
Meijer, E. J. et al. Functional connectivity in preterm infants derived from EEG coherence analysis. Eur. J. Paediatr. Neurol. 18, 780–789 (2014).
González, J. J. et al. Assessment of electroencephalographic functional connectivity in term and preterm neonates. Clin. Neurophysiol. 122, 696–702 (2011).
McLaren, J., Holmes, G. L. & Berg, M. T. Functional connectivity in term neonates with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia. Pediatr. Neurol. 94, 74–79 (2019).
McAnulty, G. et al. School-age effects of the newborn individualized developmental care and assessment program for preterm infants with intrauterine growth restriction: preliminary findings. BMC Pediatr. 13, 25 (2013).
Myers, M. M. et al. Family Nurture Intervention in preterm infants alters frontal cortical functional connectivity assessed by EEG coherence. Acta Paediatr. 104, 670–677 (2015).
American Electroencephalographic Society guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 8, 200–202 (1991).
Shellhaas, R. A. et al. The American Clinical Neurophysiology Society’s guideline on continuous electroencephalography monitoring in neonates. J. Clin. Neurophysiol. 28, 611–617 (2011).
Fox, M. Time for Bed: HMH Books for Young Readers (HMH, 1997).
Wood, A. The Napping House: HMH Books for Young Readers (HMH, 2015).
Welch, P. D. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. AU-15, 70–73 (1967).
Nolte, G. et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 115, 2292–2307 (2004).
Wirth, L. et al. Effects of standardized acoustic stimulation in premature infants: a randomized controlled trial. J. Perinatol. 36, 486–492 (2016).
Kaffashi, F., Scher, M. S., Ludington-Hoe, S. M. & Loparo, K. A. An analysis of the kangaroo care intervention using neonatal EEG complexity: a preliminary study. Clin. Neurophysiol. 124, 238–246 (2013).
Levy, J. et al. Impact of hands-on care on infant sleep in the neonatal intensive care unit. Pediatr. Pulmonol. 52, 84–90 (2017).
Acknowledgements
The authors deeply appreciate the infants and parents for their interest in research and enthusiasm for this work. They also thank the clinical research coordinators and sleep technologists, whose tireless attention to detail and care for each of the participating infants was integral to this study. This study was supported by NIH and the Michigan Institute for Clinical and Health Research (R21HD083409; UL1TR002240).
Author information
Authors and Affiliations
Contributions
R.A.S. conceptualized and designed the study, acquired and analyzed the data, drafted the initial manuscript, and approved the final version for submission. R.D.C. assisted with study design and interpretation of the results, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. J.D.E.B. assisted with study design and interpretation of the results, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. F.H interpreted the research polysomnograms, assisted with interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. M.D.C. assisted with interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. J.W.B. analyzed the data, assisted with interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
R.A.S’ research is supported by grants from NIH, PCORI, the Pediatric Epilepsy Research Foundation, and the University of Michigan. She serves as a consultant for the Epilepsy Study Consortium and as an Associate Editor for Neurology. She receives royalties from UpToDate for authorship of topics related to neonatal seizures. R.D.C. has had financial relationships with the American Academy of Sleep Medicine, the Associated Professional Sleep Societies, UpToDate, and Cambridge University Press; has been a member of the boards for the International Pediatric Sleep Association and the not-for-profit Sweet Dreamzzz; is a member of an advisory board for the not-for-profit Pajama Program; receives research support from the NIH; is named in patents and copyrighted material, owned by the University of Michigan, that concern identification and treatment of sleep disorders; and has received royalties from Zansors. J.D.E.B. receives grant funding from NIH. F.H. has previously served as a consultant for Biogen, and has received research funding from Jazz pharmaceuticals. M.D.C. has nothing to disclose. J.W.B. receives grant funding from NIH and is named in patents, owned by Michigan Technological University and the University of Michigan, that concern identification and treatment of sleep disorders.
Consent statement
A parent of every infant provided written informed consent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shellhaas, R.A., Chervin, R.D., Barks, J.D.E. et al. Lateralized neonatal EEG coherence during sleep predicts language outcome. Pediatr Res 91, 962–969 (2022). https://doi.org/10.1038/s41390-021-01554-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01554-y
This article is cited by
-
QEEG findings in nonsyndromic sagittal craniosynostosis
Scientific Reports (2024)