Abstract
Background
Androgens control rodent inguinoscrotal testicular descent during a “programming window” (E12–17). It is proposed that androgen masculinises the genitofemoral nerve, but the mechanism remains unknown. We investigate androgen receptor (AR)-containing target organs: inguinal fat pad (IFP) and mammary bud (MB), supplied by the genitofemoral nerve, hypothesizing that neurotrophic factors may retrogradely masculinise the GFN.
Methods
The IFP, MB and bulbocavernosus (BC) muscle were collected at E12.5/E17.5 from androgen receptor knockout (ARKO) mice and wild-type (WT) littermates. Immunofluorescence and gene expression (RT-qPCR; n = 8/group) for Bdnf, active (TrkB) and inactive (truncated TrkB) receptors, Cntf and Cntf receptor were performed.
Results
In the IFP at E12.5, ARKO TrkB mRNA expression was significantly downregulated compared to WT males (p < 0.0026). By E17.5, there was increased Bdnf expression (p < 0.0233). The MB had no differences at E12.5 and had regressed in WT males by E17.5. The BC had no differences at E12.5, but at E17.5 had significant upregulation of Bdnf expression in ARKO, compared to WT males. There were no differences in CNTF or CNTF receptor expression.
Conclusions
Androgen alters active TrkB and Bdnf expression in the IFP. IFP Bdnf signalling may regulate “masculinisation” of the GFN sensory nerves to indirectly control inguinoscrotal testicular descent.
Impact
-
Androgen mediates neurotrophin release in the inguinal fat pad in mice, which may facilitate normal testicular descent by masculinising the GFN by peripheral uptake of neurotrophin.
-
This is the first study to examine the role of neurotrophins in testicular descent.
-
This suggests novel steps in the mechanical process of normal testicular descent that may be abnormal in some children with undescended testes.
Similar content being viewed by others
Introduction
Testicular descent occurs in two distinct phases, controlled by different hormonal cues. The first stage, transabdominal descent, is controlled by insulin-like hormone 3. The second stage, inguinoscrotal descent, is controlled by androgen in both humans and animal models1. Inguinoscrotal descent is incompletely understood; however, >90% of undescended testis in humans occur due to a failure of inguinoscrotal descent. Previous research has suggested that androgen may masculinise the sensory branch of genitofemoral nerve (GFN) and the surrounding tissues, including the gubernaculum (genito-inguinal ligament), which was historically thought to control the primary effect of androgen. However, the question of how androgen primarily acts to cause these changes in the GFN remains unanswered. We propose neurotrophins are the missing link, with an indirect mechanism similar to that found in the neighbouring bulbocavernosus (BC) muscle.
The bulbocavernosus muscle is a sexually dimorphic structure located deep to the scrotum and supplied by the pudendal nerve. Androgen controls the masculinisation of this muscle by an indirect mechanism. First, androgen stimulates the muscle cells at the end of the nerve to produce neurotrophic factors, such as brain-derived neurotrophic factor (Bdnf) and ciliary neurotrophic factor (Cntf). These neurotrophic factors (BDNF and CNTF) are then are taken up by the nerve endings in the muscle and masculinise all of the motor branches of the pudendal nerve2. As the pudendal nerve is masculinised by BDNF and CNTF in response to androgen, we considered that GFN may be masculinised by the same mechanism.
Unlike the pudendal nerve, the GFN cannot be masculinised via the muscle it supplies, the cremaster muscle, as the cremaster muscle does not express androgen receptors (ARs). Additionally, ARs are not present in the GFN sensory cell bodies or the gubernaculum itself during the “androgen-programming window” of E12.5–16.5 in mice (day 0.5 = vaginal plug)3,4. However, there are ARs in the inguinal fat pad (IFP) and the inguinal mammary bud (MB), which are located in the connective tissue between the groin and scrotum5. Both the IFP and MB are innervated by sensory branches of the GFN, and research from our laboratory has shown that the sensory branches of the GFN are sexually dimorphic and supply the IFP before testicular descent6. As ARs are located in the IFP and MB (but not the GFN sensory nucleus) during the androgen-programming window7, we hypothesised that the mesenchyme of the IFP or MB may respond to androgen stimulation by producing a neurotrophin, which then masculinises the GFN.
Following GFN masculinisation in rodents, secondary changes occur in innervated areas, including the gubernaculum and the cremaster muscle6,8,9. The masculinised sensory branches of the GFN produce the neurotransmitter calcitonin-gene-related peptide (CGRP), which then stimulates growth in the gubernaculum and provides a chemotactic gradient to direct gubernacular migration9. In the humans, it is not yet known whether it acts in the same way, but it is known that exogenous CGRP can trigger closure of an inguinal hernia in vitro, consistent with a key role for the GFN and CGRP in human testicular descent and inguinal hernia closure10,11. Knockout mouse models of CGRP eliminate alpha CGRP and do not exhibit undescended testes. Beta CGRP is coded by a different gene and has 90% homology. This makes it difficult to exclude a role for CGRP in testicular descent.
BDNF production is known to be androgen dependent and neuroprotective12,13,14,15. In the mammary gland, Bdnf expression is virtually identical in male and female foetuses until E13. At E13.5, androgen causes Bdnf to be sequestered by an inactive, truncated form of the Bdnf receptor (truncated TrkB), to prevent Bdnf–TrkB signalling in sensory fibres16. TrkB expression changes to the truncated form and causes sensory axon pruning from the male gland beginning at early E13. Many isoforms of Bdnf exist, but primers targeting a conserved region between isoforms were chosen. As the BC muscle also produces Bdnf to masculinise the pudendal nerve, we included the BC muscle as a positive control tissue. Therefore, we investigate neurotrophins and the effect of androgens in the IFP, MB and BC muscle in embryonic mice, which could cause masculinisation of the GFN.
Materials and methods
Animals
AR knockout (ARKO) mice were supplied by Austin Health, Melbourne, Australia. Genetic modification of the third exon of the AR gene containing the DNA-binding domain was targeted by the Cre/loxP system to remove 1114 bp, rendering the animal completely insensitive to androgens17. All experiments were performed with approval from Murdoch Children’s Research Institute animal ethics committees (AEC no. A854). Mice were fed normal chow and housed in enriched, temperature-controlled environment of 23 °C and 44% humidity, with a 14-h light and 10-h dark cycle. Genotyping of the ARKO mice can be found in Supplementary Methods Section 1.1. Mice were genotyped and their sex determined before experimental breeding and downstream analysis (Supplementary Methods Section 1.2).
Experimental ARKO mice
Females with one copy of the mutation on the AR gene X chromosomes (het) were bred with wild-type (WT) males to produce litters containing ARKO males. Pregnant dams were sacrificed and foetuses were collected via hysterectomy at embryonic day 12.5 and 17.5 (vaginal plug = day 0.5), which is at the beginning and end of the androgen-programming window17. Dams were sacrificed by anaesthesia with isoflurane gas, followed by cervical dislocation. Embryos were removed from the uterus and placed on ice for 15–20 min before decapitation, and then the BC, lower abdominal skin containing the mammary line and MB and IFP were collected from the embryos and snap frozen for downstream analysis.
Standard histology
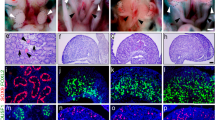
Pelvis from a foetus at embryonic day 17.5 were fixed in 4% paraformaldehyde at 4 °C overnight before being processed through graded alcohols and xylene and embedded in paraffin. Samples were sectioned in the sagittal plane at 5 µm and floated on silane-coated slides. Slides were stored at room temperature (RT) for minimum 24 h before being stained (Fig. 1).
a Schematic illustrating the distribution of androgen receptors (magenta) in male mice. b Immunohistochemistry demonstrating the distribution of androgen receptors in the male mouse. (Reprinted with permission from Allnutt et al.9).
Immunofluorescence
Sections were heated in the microwave (5 min) in 0.01 M citric acid buffer (pH 7.0) for antigen retrieval, then incubated for at least 2 h in blocking buffer (10% bovine albumin and 10% horse serum in phosphate-buffered saline (PBS)) at RT.
Sections were incubated overnight at 4 °C with primary antibodies against BDNF and active tropomyosin receptor kinase B (TRKB) were diluted in 1:5 dilution of blocking solution in 0.1% PBS triton (blocking solution: 10% horse serum, 5% bovine serum albumin and PBS 0.1% triton). Slides were rinsed with PBS and secondary antibodies were applied for 2 h at RT (Supplementary Table 1). Cell nuclei were counterstained with DAPI (4,6-diamidino-2-phenylindole). After a final PBS wash, slides were mounted with Mowiol, coverslipped and stored at 4 °C in the dark until confocal imaging. Positive and negative control tissue, mouse heart muscle, rat sciatic nerve and adult female mouse MB was established for BDNF, TRKB and truncated TRKB and included with each sample staining procedure (Supplementary Methods Table 2).
Confocal imaging
Sections of the MB, IFP and BC muscle were imaged on the Dragonfly spinning disc confocal microscope and images were acquired via the Fusion Software (version 2.0, Andor, Northern Ireland). Primary antibodies against BDNF and its active receptor TRKB were used (Supplementary Methods Table 2) along with secondary antibodies and DAP1 (1:5000 in PBS) were used to label all nuclei. Confocal images were captured at ×40 and ×60 magnifications. Laser at 488 mm for BDNF and 637 mm excited the DAP1 and Alexa 568 for active TRKB, respectively, to create merged images, which were edited with the Fiji Image J software (version 1.50; LOC1, University of Wisconsin-Madison, Madison, WI, USA) for colour, brightness, contrast correction and scale bar inserted in Fig. 3.
Quantification of immunofluorescence
The quantitative analyses of BDNF and TRKB active protein was performed using Image J (Fiji version 1.52i USA). The average intensity of the BDNF (green channel) and TRKB (red channel) was taken from three independent mice from the WT and ARKO at E17.5. Using Image J, the three-channel image was separated into three colours (red, green and blue) within the IFP and the BC where the area was kept the same. Threshold value was set and kept consistent during each colour measurement between the genotypes and the mean of the protein intensity was calculated using the intensity (without background) over the region of interest. The IFP and MB were regions of interest, with the BC included as a positive control.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the IFP, BC and MB of ARKO and WT male and female embryos at E12.5 and E17.5 using the RNeasy mini column (Qiagen, Cat:74104). RNA was treated with DNase I (Qiagen, Cat: 79254) and the concentration was determined using NanoDrop spectrophotometer. Gene expression was measured using 10 ng/µl of cDNA by GoTaq qPCR (Promega, Cat: A6001) for RT-qPCR. Nucleotide sequences for the neurotrophins (Supplementary Methods Table 3) were used and the expression was normalised to Rpl32. Rpl32 is a housekeeping gene that enables normalisation for heterogeneity in clinical samples, as well as for variability introduced during RNA extraction and cDNA synthesis18. Statistical analysis was performed using Prism v7.04 (GraphPad Software). Two-way analysis of variance was used to analyse for treatment effects and timepoints. When appropriate, a post hoc Tukey’s multiple comparison test was performed. Comparison between treatment and control was analysed by Student’s paired t test, and a p value of <0.05 was considered significant.
Results
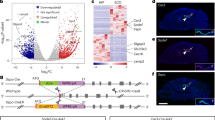
In the IFP at E12.5 days, expression of active TrkB was downregulated in both ARKO male mice and females (WT), compared to the WT male counterpart (p = 0.0026, p = 0.0308); Fig. 2a). At E17.5, Bdnf expression was upregulated in ARKO males and WT females compared to the WT male control (p = 0.0233, p = 0.0099; Fig. 2b), consistent with upregulation of Bdnf in response to downregulation of the active TrkB receptor. There were no differences in the expression of Cntf or its receptor between WT and ARKO males at both timepoints.
Real-time qPCR was used to demonstrate gene expression changes of Bdnf, along with its active and non-active TrkB receptor and Cntf with its receptor at embryonic day a E12.5 in the inguinal fad pad (IFP), b E17.5 (IFP), c E12.5 in the mammary bud (MB), d E17.5 (MB), e E12.5 in the bulbocavernosus (BC) and f E17.5 in the (BC). Black bars illustrate wild-type male mice, grey bars represent the androgen receptor knockout male mice, and white bars illustrate wild-type female mice. Standard error of mean was used and p values of <0.05 (*) and <0.01 (**) were considered significant. Tukey ANOVA and Student’s t test if significance was determined. N = 8 per genotype. 166 × 262 mm (300 × 300 DPI).
At E12.5 and E17.5 days in the inguinal MB, there were no significant differences in the expression of Bdnf and its receptors or Cntf and its receptor (Fig. 2c, d). There were no differences in the expression of Bdnf and its receptors or Cntf and its receptor at the two timepoints examined.
In the BC, a known sexually dimorphic muscle, there were no differences between WT and ARKO males in the expression of Bdnf, its active receptor or its inactive truncated receptor at E12.5 days (Fig. 2e). Similarly, Cnft and its receptor expression were similar between WT and ARKO males. However, by E17.5, Bdnf expression was upregulated in ARKO males compared to WT males (p = 0.0066) and in WT females compared to WT males (p = 0.0060). This upregulation in both ARKO and WT females was also observed in the active Bdnf receptor but was not statistically significant (Fig. 2f). The expression of Cntf and its receptor did not demonstrate any difference between WT male, ARKO males and WT females.
Immunofluorescence at E17.5 showed that ARKO male mice expressed significantly increased TRKB active receptor (red) when compared to the WT male mice, with trend to an increase in BDNF protein expression (green) (Fig. 3). The fluorescence intensity per area of the IFP and BC show a significant increase in both BDNF and active TRKB receptor in the ARKO mice when compared to the WT M (Fig. 4). The BC tissue was used as a positive control.
Overlay immunohistochemistry of the pelvis of both ARKO male and WT male mice at ×10 magnification. Blue is DAPI, green is BDNF and red is full-length TRKB (active). Individual images at ×10 magnification were stitched together using the Image J Stitching plugin, created by Preibisch et al.25. Scale bar equals 100 mm (imaged with ×10 magnification). The inguinal fat pad (IFP) and bulbocavernosus (BC) were taken at ×60 oil magnification, blue is DAPI, green is BDNF and red is full-length TrkB (active) with a merged image in the last panel. Scale bar equals 10 μm (imaged with ×60 magnification).
Bar graph demonstrates the average fluorescence intensity of BDNF and its active receptor TRKB. Intensity was calculated in IFP and BC using n = 3 images and keeping the area of interest the same in all sections. Backgrounds were removed and intensity was measured. Standard deviation was used and a p value of <0.001 (***) was considered significant. Student’s t test was used for statistical analysis.
Discussion
Quantitative analysis of gene expression by qPCR in this study showed that in the IFP androgen knockout is associated with a significant downregulation of the receptor for Bdnf, active TrkB, compared to WT males (and similar to females), at the start of the androgenic-programming window19. There was also a trend to increased Bdnf expression in ARKO males at E12.5, but this was not significant. By E17.5, Bdnf expression in ARKO males and WT females was significantly increased compared with WT males. This was observed in the mRNA analysis as well as in the immunofluorescence. As in most cellular signalling systems, there is some feedback between agonist and receptor, and the increased Bdnf expression in ARKO males and WT females at E17.5 is consistent with possible loss of negative feedback with the lack of a receptor. Alternatively, turnover of Bdnf and Trkb may have been impaired by androgen blockade. As the sensory nerve endings present in the IFP are branches of the genital branch of the GFN8, this suggests that masculinisation of the GFN sensory fibres may be controlled by peripheral uptake of Bdnf from the IFP.
In the MB, androgen blockade did not reveal any changes in gene expression of Bdnf, Cntf or their receptors at either E12.5 or E17.5. In retrospect, the time points chosen may have missed some differences, as differences in Bdnf expression were not seen by other authors until E13.0, and E17.5 is after complete regression of the MB in WT males.
In the BC, there were no obvious differences in gene expression at E12.5, but by E17.5 there was more Bdnf (and probably active TrkB) expression in ARKO males and WT females compared to WT males.
Androgen manipulation is known to differentially affect Bdnf and Cntf levels in a structure-dependant manner. Our results in the BC confirm published studies demonstrating that androgen knockout causes increased Bdnf in the BC muscle12. The level of BDNF protein in muscles can be altered through either changes in production or retrograde transport20,21. The regression of MB in WT males and persistence of MBs in ARKO male animals confirms that androgen blockade causes a persistence of MBs16, which may be involved in interfering with testicular descent3.
The IFP is known to be supplied by the GFN and contains AR during the androgenic-programming window in the rodents5, consistent with it being a target organ for androgens during sexual differentiation. During inguinoscrotal testicular descent, the extracellular matrix (ECM) in the IFP of the male undergoes significant remodelling, with resorption of the ECM to make a clear space around the migrating gubernaculum to enable the testes to reach the scrotum unobstructed22. It is not known whether the IFP makes the enzymes to remodel the ECM or whether they are produced by the gubernaculum itself. Either way, the IFP is clearly an important target for androgenic stimulation during testicular descent, and the changes in expression of neurotrophins and their receptors in response to androgen are likely to be a part of this process. However, whether the neurotrophins modulate the sensory nerve endings of the GFN remains an assumption at this time in our knowledge and would require further study.
The existing literature on the role of neurotrophins examines the effect of androgen on Bdnf expression in the brain and the spinal nucleus of the BC12,21. However, the recent recognition that some patients with WAGR (Wilms tumour, anirida, genital anomalies and mental retardation), where there is a deletion on chromosome II, often have haploinsufficiency of BDNF suggests a possible link between undescended testis and deficiency in BDNF function23. There has been limited investigation into the role of neurotrophins in testicular descent outside our laboratory. In addition, our experiments are limited to gene expression within tissues, rather than protein expression. We were also limited by the time period over the programming window and have only examined the beginning and the end of this window. The changes that happen within the window have not been examined. We have also demonstrated changes within sexually dimorphic tissues; however, we have not proven these changes actually act on the GFN, and we are assuming that these neurotrophins modulate the GFN from the existing literature. We also have not directly proven that Bdnf triggers CGRP, and recent literature suggests that Bdnf may respond to androgen blockade by increased expression, consistent with a suppressive role on nerves in the male24. Also, the interaction between Bdnf and Cntf is an evolving area, and whether androgen primarily affects Bdnf is unknown in the IFP. We also presume that Cntf and Bdnf interaction is similar to the results of Liu et al.16, where Cntf has secondary changes following the primary response of Bdnf to androgen.
Conclusion
This study suggests that the expression of Bdnf and its receptor, TrkB, in the IFP during the embryonic androgen-programming window is androgen dependent. These results suggest that the masculinisation of the GFN sensory fibres in the rodent may be controlled by changes in the peripheral uptake of Bdnf from the IFP to control testicular descent. If this indirect mechanism for androgenic stimulation also occurs in the humans, it would provide further ways that defects in the process may lead to cryptorchidism.
Disclaimer
This is original work, which has not been published or submitted for publication elsewhere.
References
Hutson, J. M. A biphasic model for the hormonal control of testicular descent. Lancet 2, 419–421 (1985).
Churchill, J. A. et al. Gubernaculum as icebreaker: do matrix metalloproteinases in rodent gubernaculum and inguinal fat pad permit testicular descent? J. Pediatr. Surg. 46, 2353–2357 (2011).
Nation, T. R. et al. The effect of flutamide on expression of androgen and estrogen receptors in the gubernaculum and surrounding structures during testicular descent. J. Pediatr. Surg. 46, 2358–2362 (2011).
Allnutt, B. et al. The common fetal development of the mammary fat pad and gubernaculum. J. Pediatr. Surg. 46, 378–383 (2011).
Balic, A. et al. Hidden in plain sight: the mammary line in males may be the missing link regulating inguinoscrotal testicular descent. J. Pediatr. Surg. 45, 414–418 (2010).
Schwindt, B., Farmer, P. J., Watts, L. M., Hrabovszky, Z. & Hutson, J. M. Localization of calcitonin gene-related peptide within the genitofemoral nerve in immature rats. J. Pediatr. Surg. 34, 986–991 (1999).
Vigueras, R. M., Moreno-Mendoza, N., Reyes, G. & Merchant-Larios, H. Androgen receptor and calcitonin gene-related peptide in neurons of the genitofemoral nerve during testicular descent induced with human chorionic gonadotropin. Arch. Med. Res. 34, 166–170 (2003).
Larkins, S. L. & Hutson, J. M. Fluorescent anterograde labelling of the genitofemoral nerve shows that it supplies the scrotal region before migration of the gubernaculum. Pediatr. Surg. Int. 6, 167–171 (1991).
Shenker, N. S., Huynh, J., Farmer, P. J. & Hutson, J. M. A new role for androgen in testicular descent: permitting gubernacular cell proliferation in response to the neuropeptide, calcitonin gene-related peptide. J. Pediatr. Surg. 41, 407–412 (2006).
Hutson, J. M. et al. In vitro fusion of human inguinal hernia with associated epithelial transformation. Cells Tissues Organs 166, 249–258 (2000).
Hutson, J. M. et al. The regulation of testicular descent and the effects of cryptorchidism. Endocr. Rev. 34, 725–752 (2013).
Verhovshak, T., Cai, Y., Osborne, M. C. & Sengelaub, D. R. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology 151, 253–261 (2010).
Verhovshak, T., Rudolph, L. M. & Sengelaub, D. R. Brain-derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience 2, 103–114 (2013).
Kishino, A., Ishige, Y., Tatsuno, T., Nakayama, C. & Noguchi, H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp. Neurol. 144, 273–286 (1997).
Yang, L. Y., Verhovshek, T. & Sengelaub, D. R. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology 145, 161–168 (2004).
Liu, Y. et al. Sexually dimorphic BDNF signalling directs sensory innervation of the mammary gland. Science 338, 1357–1360 (2012).
Notini, A. J., Davey, R. A., McManus, J. F., Bate, K. L. & Zajac, J. D. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J. Mol. Endocrinol. 35, 547–555 (2005).
Kriegova, E. et al. PSMB2 and RPL32 are suitable denominators to normalize gene expression profiles in bronchoalveolar cells. BMC Mol. Biol. 9, 69 (2008).
Welsh, M., Suzuki, H. & Yamada, G. The masculinization programming window. Endrocr. Dev. 27, 17–27 (2014).
Watson, F. L. et al. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J. Neurosci. 19, 7889–7900 (1999).
Fargo, K. N., Foster, A. M., Harty, M. W. & Sengelaub, D. R. Estrogen alters excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. J. Neurobiol. 56, 66–77 (2003).
Heyns, C. F. The gubernaculum during testicular descent in the human fetus. J. Anat. 153, 93–112 (1987).
Han, J. C. et al. Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behaviour and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex 49, 2700–2710 (2013).
Chan, C. B. & Ye, K. Sex differences in brain-derived neurotrophic factor signaling and functions. J. Neurosci. Res. 95, 328–335 (2017).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
Acknowledgements
We would like to thank Dr. Amanda Vannitamby and Dr. Ruili Li from Surgical Research Laboratory for their assistance with animal breeding and processing; Desai from Andrology Laboratory, ANZAC Research Institute, Sydney, NSW, Australia for assistance in hormone analysis; Ms. Louise O’Connor and Dr. Trevelyan Menheniott from GRIP Laboratory, MCRI for advice on qPCR; and Shirley D’Cruz for assistance in preparation of the manuscript. The authors gratefully acknowledge the help of Mark Jimenez and Reena. This research was supported by NHMRC Grant 1049014 and the Victorian Government’s Operational Infrastructure Support Program. Financial support was provided by the Australian National Health and Medical Research Council (Grant Number APP1144752).
Author information
Authors and Affiliations
Contributions
All listed authors contributed substantially to conception and design of this study. J.V., G.S. and L.O. contributed to the acquisition of data and analysis of data. J.V., G.S. and J.M.H. contributed to the interpretation of data, drafting the initial article and subsequent revisions. All listed authors contributed to the revision of the article and critical appraisal of content, as well as approving the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
Patient consent was not required for this research.
Ethical approval
Ethical approval was obtained prior to undertaking.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Vikraman, J., Sarila, G., O’Conner, L. et al. BDNF is upregulated by androgen in the inguinal fat pad of immature mice and may regulate inguinoscrotal testicular descent. Pediatr Res 91, 846–852 (2022). https://doi.org/10.1038/s41390-021-01458-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01458-x